![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
21 Cards in this Set
- Front
- Back
|
IR group: OH (alcohol) cm-1 and characteristics |
3300-3600 Strong, broad |
|
|
IR group: sp2 CH cm-1 and characteristics |
3000-3100 Weak-med, sharp |
|
|
IR group: sp3 CH cm-1 and characteristics |
2800-3000 Weak-med, sharp |
|
|
IR group: O=CH (aldehyde) cm-1 and characteristics |
2700-2850 Pair, weak-med |
|
|
IR group: C=O cm-1 and characteristics |
1670-1800 Strong, sharp |
|
|
NMR: Saturated methyl shift |
0.7 - 1.8 ppm |
|
|
NMR: Alkoxy/ Alkyl halide shift |
3.0 - 4.5 ppm |
|
|
NMR: Hydroxyl shift |
1.5 - 5.0 ppm Integrates to 1H |
|
|
Difference between extraction and washing |
Extraction draws good material from an impure matrix Washing removes impurities from good material |
|
|
Why should you recrystallize slowly? |
Slower cooling gives better crystals with fewer trapped impurities |
|
|
Which components experience the most mobile phase during TLC? |
Nonpolar; they interact the least with the polar stationary phase (silica gel) |
|
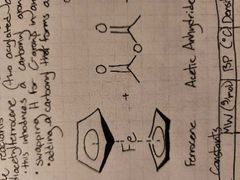
What occurs? (Reactants, solvent, prod) |
Acetylferrocene and acetic anhydride react with a phosphoric acid catalyst to produce acetylferrocene and acetic acid +ketone formed from acetone |
|
|
Unlike the other reactions, dehydration of an ester is catalyzed by the addition of... |
A base (NaOH) |
|
|
In aqueous solutions, carboxylic acids are ___ and carboxylate salts are ___. |
Soluble; insoluble |
|
|
What is a positive result for a KMnO4 desaturation test? |
Purple KMnO4 forms brown precipitates |
|
|
What is a positive result for a Br2 desaturation test? |
Red-orange Br reacts out; soln returns to colorless |
|
|
What is the purpose of reflux? |
Accelerate rxn rate and conserve solvent |
|
|
What does IR tell you? |
The types and nature of bonds in a compound |
|
|
What does TLC tell you? |
The number of components, relative amounts of components, and helps identify components (versus a standard) |
|
|
What is the purpose (and method) of recrystallization? |
|

