![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
29 Cards in this Set
- Front
- Back
|
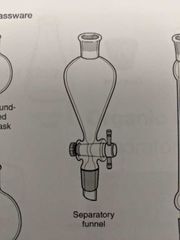
Separatory funnel |
|
|
|
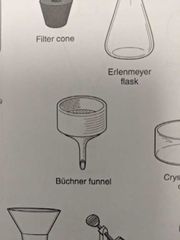
Buchner funnel |
|
|
|
Filter flask |

|
|
|
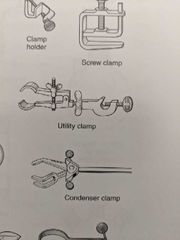
Clamp |
|
|
|
Melting point tube |

|
|
|
Vacuum filtration |

|
|
|
Recrystallization steps |
-select a proper solvent -heat the solvent to its boiling point -dissolve the solid compound in the minimum amount of boiling solvent -filter the hot mixture -cool the mixture -collect the crystals by suction filtration |
|
|
Extraction |

|
|
|
Short path distillation |

|
|
|
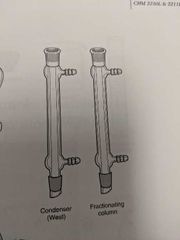
Condenser |
|
|
|
Sodium fusion |
-add 1 sodium sphere in dry test tube -heat until vapors rise about 1/3 way up. Will have red glow at the bottom of the tube. -remove from flame. Add 10 mg of unknown solid or 2 drops of unknown liquid. -heat again. Add 10 mg of unknown again. Heat for at least 3 minutes. -while hot, plunge into a 50 ml beaker containing 15 ml of distilled water. -heat the contents and gravity filter |
|
|
Neutralization equivalent |

|
|
|
Percent yield |

|
|
|
Percent recovery |

|
|
|
2-4 dinitrophenylhydrazine test |
Presence of aldehydes & ketones Positive= yellow/orange/red |
|
|
Iodoform test |
Presence of methyl ketones Positive=yellow ppt |
|
|
Tollen's reagent |
Presence of aldehydes positive= silver mirror or black ppt |
|
|
Chromic acid test |
Presence of aliphatic or aromatic aldehyde Positive=green ppt Aliphatic aldehyde= <30 seconds Aromatic aldehyde= >30 seconds |
|
|
Schiff's reagent |
Presence of aldehydes Positive=magenta |
|
|
Lucas test |
Distinguish 1/2/3 alcohol 1=NR 2=milky within 5 mins 3=white,cloudy immediately |
|
|
Jones oxidation |
Only 1 and 2 alcohols react (orange --> green) |
|
|
Iodoform test |
Presence of 2 alcohol=yellow ppt |
|
|
Ceric ammonium nitrate |
1/2/3 alcohols=red Phenols=green/brown ppt |
|
|
Hinsberg test |
Presence of 1/2 amines 1 amine= 1 layer 2 amine= 2 layers 3 amine= 2 layers--> turns into 1 layer after |
|
|
Solubility test for amines |
Soluble in HCl=amine Insoluble in H2O=aromatic amine |
|
|
Silver nitrate |
Presence of carboxylic acids |
|
|
Bromine in methylene chloride |
presence of hydrocarbons Positive=colorless Negative=light brown |
|
|
Baeyer test |
Presence of alkene or alkyne Positive=brown Negative=dark pink |
|
|
Belstein test |
Presence of halogen Burn w/ copper wire Positive=green flame |

