![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
42 Cards in this Set
- Front
- Back
|
what are enzymes |
biological catalysts that speed up chemical reactions |
|
|
why are enzymes useful in small amounts |
reused repeatedly and are therefore effective in small amounts |
|
|
what is activation energy |
in an chemical reaction a certain amount of energy needs to be supplied to chemicals before the reaction will start |
|
|
describe 2 ways enzymes work |
2 substrates molecules need to be joined being attached tot he enzyme holds them close together- reducing repulsion enzyme is cataylisng a breakdown reaction fitting into active site puts strain on bonds in substrate |
|
|
what is the active site |
a specific region of enzyme is functional |
|
|
what is the substrate |
moleuclue on which the enzyme acts is called the substrate this fits into the active site and forms an enzyme-substrate complex |
|
|
describe the induced fit model |
the active site is able to change shape so the substrate molecule can fit in |
|
|
describe the lock and key model |
the active site is rigid and cannot change shape so the substrate needs to be complementary |
|
|
describe to 2 ways to measure enzyme activity |
how fast the product is made-measure the amount of end product present at different times during the reaction rate can be calculated how fast the substrate is broken down- measure amount of substance molecules left at different times during the experiment the reaction rate can be calculating |
|
|
describe the effect of temperature on enzymes |
low temp-substrates fits into active site high temp-enzymes vibrates more this breaks some of bonds that hold it in shape higher temp-active site changes shape and substrate can no longer fit cause it's denatures |
|
|
describe the effect of pH on enzymes |
above and below the optimum H+ and OH- ions found in acids and alkails can disrupt the ionic bonds and hydrogen bonds that hold the enzyme's tertiary structure in place and enzymes becomes denatures |
|
|
describes effect of substrates concentration on enzymes |
low substrate concentration-not all active sites are occupies saturation point - all active site are occupied beyond the saturation point- all active sites are occupied and there are space substrate molecules |
|
|
describe competitive inhibitor and factors |
have similar shape to the substrate they compete with substrate for available active site if substrate concentration is increased the effect of the inhibitor is reduced they are not permanently bound to active site and when it leaves another molecule can take its place |
|
|
describe non-competitive inhibitors |
they attach themselves to the enzyme at a binding site and changes shape of active site so the enzyme can't function so increasing substrate concentration makes no difference |
|
|
function of DNA |
it's used to store your genetic information |
|
|
function of RNA |
transfer genetic information from DNA to ribsomoes |
|
|
what is a nucleotide and what does it look like |
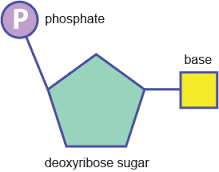
it is a type of biological moleclue |
|
|
how is phosphodiester bond formed |
group of 1 nucleotide and sugar of another nuceltoide are joined together |
|
|
what is a sugar phosphate backbone |
chain of phosphates and sugars |
|
|
describe DNA strucutre |
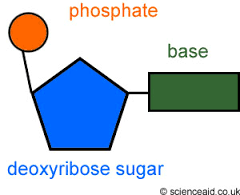
double helix structure strands are polynucleotide really long and coiled up very tightly and a lot of genetic information can fit into small space 2 DNA polynucleotides strands join together by H-bonds between the bases adenine-thymine (2 H bonds) guanine -cytosine (3 H bonds) 2 polynucleotides strands are antiparallel and run opposite directions |
|
|
describe DNA adaptions |
large molecule and carries lots of genetic information base paring leads to DNA being able to replicate and transfer information as mRNA very stable structure which normally passes from generation to generation without change 2 separate strands are joined only with H-bonds which allow them to separate during DNA replication and protein synthesis |
|
|
describe structure of RNA |
single, short single strand pentose sugar is ribose adenine uracil guanine cytosine |
|
|
describe DNA replication |
1.DNA helicase breaks hydrogen bonds between bases on 2 polynucleotide DNA strands. This makes helix unwind to 2 single strands 2. each original single acts as a template for a new strand free-floating DNA nucleotides are attracted to their complementary exposed bases on each original template strand 3.condensation reactions joins the nucleotides of new strand together by DNA polymearse hydrogen bongs between the bases on the original and new strands 4. each new DNA molecule contain one strand from original DNA molecule and 1 new strand this is the semi-conservative method |
|
|
describe the conservative model |
The complete parent DNA molecule acts as a template for the new daughter molecule, which is assembled from new nucleotides. The parent molecule is unchanged.
|
|
|
describe the semi-conservative model |
The parent DNA molecule separates into its two component strands, each of which acts as a template for the formation of a new complementary strand. The two daughter molecules therefore contain half the parent DNA and half new DNA (semi-conservative hypothesis).
|
|
|
what is the function of ATP and what does it look like |
stores energy |
|
|
hydrolysis reaction of ATP |
ATPaseENERGY is used
ATP +H20= ADP +Pi |
|
|
condensation reaction of ATP |
ATP synthase is used
ADP +Pi= ATP + H2O |
|
|
describe the role of ATP |
releases less energy than glucose molecule energy released quickly ATP can't be stored and has to be continuously made within the mitochondria of cells |
|
|
what is ATP is used for |
metabolic processes- build up macromolecules from thier basic units movement- provide energy for muscle contraction active transport-change shape of carrier protiens inplasma memebrane secretion-form lysosmoes activation of molecules- |
|
|
describe the dipolar water molecule |
water molecule has both + and - poles so it's bipolar |
|
|
describe the hydrogen bonds in water |
+ pole of 1 water molecule will be attracted to - pole pf another water molecule so the attraction between the opposite poles is called hydrogen bonding |
|
|
describe the specific heat capacity of water |
water molecules stick together t takes more energy to separate them due to hydrogen bond water acts as a buffer against temperature change so this is helpful in aquatic environment |
|
|
describe the latent of vaporisation of water |
hydrogen bonding between water molecules this means that it requires a lot of energy to evaporate 1g of water |
|
|
describe the cohesion and surface tension in water |
molecules sticking together due the hydrogen bonding this is cohesion so water can be pulled up the xylem tube surface tension means the water surface acts like skin so some insects can walk on water |
|
|
describe metabolism in water |
it is used to break down many complex molecules by hydrolysis or used for condensation reaction chemical reaction take place in aqueous medium and water is a major raw material in photosynthesis |
|
|
describe water a solvent |
water dissolves in gases, wastes, inorganic ions, small hydrophilic molecules and enzymes |
|
|
describe other important features of water |
evaporation cools organism not easily compressed and provides support transparent so aquatic plants can photosynthesis and light rays can penetrate jelly-like fluid that fills the eye and so reach the retina |
|
|
describe the role of iron ions |
haemoglobin is a large prtien that carries oxygen it's made up of 4 different polypeptide chains each with an iron ion Fe2+ in the centre so when oxygen is bound it becomes Fe3+ until oxygen is released |
|
|
describe the role of hydrogen ions |
more H+ present lower the pH enzyme-controlled reactions are all affected by pH |
|
|
describe the role of sodium ions |
acts as a co-transport for glucose or amino acid can be transported into a cell |
|
|
describe the role of phosphate ions |
phosphate ion is attached to another molecule. bonds between phosphate groups that store energy in ATP. Phosphate groups in DNA and RNA allow nuclotides to join up to form the polynucleotides. |

