![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
48 Cards in this Set
- Front
- Back
|
What is the relative atomic mass? |
The relative atomic mass of any atom is the number of times the mass of one atom of an element is greater than 1/12 of the mass of one carbon-12 atom. |
|
|
State the formula of relative atomic mass. |
Relative atomic mass = mass of one atom of the element/ mass of 1/12 of an atom of carbon-12. |
|
|
What is the symbol for relative atomic mass? |
The symbol for relative atomic mass is Ar. |
|
|
Why relative atomic mass has no unit? |
Relative atomic mass is a ratio and therefore has no unit. |
|
|
State the Ar of hydrogen, carbon, oxygen and chlorine. |
Ar of : Hydrogen = 1 Carbon = 12 Oxygen = 16 Chlorine = 35.5 |
|
|
Explain why the Ar value of chlorine is not a whole number? |
The Ar value of chlorine is not a whole number because chlorine occur as mixtures of isotopes. Chlorine exists in 2 isotopic forms: chlorine-35 and chlorine-37. A sample of chlorine is made up of 75% of chlorine-35 and 25% of chlorine-37. Hence, relative atomic mass of chlorine = (75% × 35) + (25% × 37) = 26.25 + 9.25 = 35.5 |
|
|
How is the mass of a molecule measured? |
The mass of a molecule is measured in terms of its relative molecular mass. |
|
|
What is the relative molecular mass of an element/compound? |
The relative molecular mass(Mr) of an element or compound is the mass of a molecule, compared to 1/12 the mass of one atom of carbon-12. |
|
|
What is the formula of relative molecular mass(Mr)? |
Relative molecular mass(Mr) = mass of one molecule of an element or compound/ mass of 1/12 of an atom of carbon-12. |
|
|
Why the relative molecular mass has no unit? |
The relative molecular mass has no unit because it is a ratio. |
|
|
Calculate the Mr of ethanol (C2H5OH) |
Mr of ethanol: 46. |
|
|
What is the relative formula mass? |
The relative formula mass is the relative molecular mass of an ionic compound. |
|
|
What is the symbol of relative formula mass? |
The symbol of relative formula mass is Mr. |
|
|
Calculate the Mr of copper(II) sulfate crystals. (CuSO4.5H2O) |
Mr of copper(II) sulfate crystals is 250. |
|
|
What does the dot(.) in CuSO4.5H2O means? |
The dot(.) in CuSO4.5H2O means that there are 5 H2O molecules bonded to each CuSO4. |
|
|
What is the unit of measurement for atoms and molecules? |
The unit of measurement for atoms and molecules is the mole. |
|
|
What is the symbol of mole? |
The symbol of the mole is mol. |
|
|
A mole of substance contains the same particles as the number of atoms in 12 g of carbon-12. |
Learn by heart. |
|
|
State the Avogadro's constant. |
The Avogrado's constant states that one mole of particles (atoms,molecules, ions or electrons) contains 6× 10^23 particles. |
|
|
Equal numbers of moles contain equal numbers of particles. |
Learn by heart. |
|
|
How many hydrogen atoms are there in 3 moles of hydrogen gas? |
Hydrogen gas is made up of hydrogen molecules (H2). In one mole of H2 molecules, there are 2 moles of H atoms. In three moles of H2 molecules, there are 6 moles of H atoms.
Therefore the number of hydrogen atoms = 6 ×6 × 10^23 = 3.6 × 10^24 |
|
|
What is molar mass of an element? |
Molar mass of an element is the mass of one mole of atoms of the element. |
|
|
The molar mass is equal to the relative atomic mass (Ar) of the element in grams. |
Learn by heart. |
|
|
What is the relationship between mole and molar mass? |
Number of moles of an element = mass of element in grams/ relative atomic mass of the element. |
|
|
How many moles of lead are there in 1.204 × 10^22 atoms of lead? |
0.02 mol. |
|
|
What is the mass of 1.204 × 10^22 atoms of lead?( Ar: Pb=207) |
Mass of lead = number of moles × Ar of lead = 0.02 × 207 = 4.14 g |
|
|
One mole of a substance will have a mass equal to the relative molecular mass or relative formula mass in grams. |
Learn by heart. |
|
|
Calculate the molar mass of oxygen, iodine , magnesium oxide and water. |
Molar mass of : Oxygen: 32 g Iodine: 254 g Magnesium oxide: 40 g Water : 18 g |
|
|
How many ions are there in 20 g of magnesium oxide (MgO) ? |
Number of ions = 6 × 10^23 |
|
|
State the formula to find the percentage by mass of an element in a compound. |
Percentage by mass of an element in a compound = (Ar of element × number of atoms in formula) / relative molecular mass (Mr) of compound × 100%. |
|
|
Calculate the percentage of water in copper(II) sulfate crystals (CuSO4.5H2O). |
Percentage of water in copper(II) sulfate crystals = 36%. |
|
|
Han can we work out the formula of a compound? |
We can conduct experiments to find out the formula of a compound. 1) First, we find out the mass of the reactants taking part in the reaction. 2) Next, we work out the relative numbers of moles of the reactants used. |
|
|
Describe an experiment to work out the formula of magnesium oxide produced by the combustion of magnesium. |
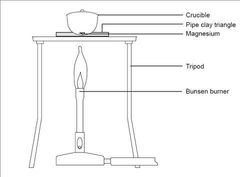
Experiment to work out the formula of magnesium oxide produced by the combustion of magnesium;
Procedure 1) Weight a crucible together with the lid. 2) Put a coil of magnesium ribbon in it and weight again. 3) Put the lid on the crucible and heat the crucible gently. When the magnesium catches fire (a white glow will be seen through the crucible), heat it more strongly. 4) Use a pair of tongs to lift the lid slightly from time to time to allow air in. Quickly replace the lid to make sure that magnesium oxide formed does not escape. 5) When the burning is complete, allow the crucible to cool. 6) Weight the crucible together with the lid and the magnesium oxide in it.
Sample results; Mass of crucible + lid = 26.52 g Mass of crucible + lid + magnesium = 27.72 g Mass of crucible + lid + magnesium oxide = 28.52 g
Calculations : Mass of magnesium = 27.72 - 26.52 = 1.20 g Mass of magnesium oxide produced = 28.52 - 26.52 = 2.00 g Mass of oxygen reacted = 2.00 - 1.20 = 0.80 g Number of moles: Mg : 1.20/24 = 0.05 O : 0.80/16 = 0.05
Molar ratio (divide by smallest number from number of moles) Mg ; 0.05/ 0.05 =1 O; 0.05/0.05 = 1
Thus the formula = MgO.
|
|
|
What is the emperical formula? |
The emperical formula of a compound shows the types of elements present in it and the simplest ratio of the different types of atoms in it. |
|
|
What is the molecular formula? |
The molecular formula is the formula that shows the exact number of atoms of each element in a molecule. |
|
|
What is the relationship between emperical formula and molecular formula? |
The molecular formula of a compound is a multiple of its emperical formula. |
|
|
State the formula to find the molecular formula. |
If emperical formula = AxBy , molecular formula = (AxBy)n (where n= 1,2,3,etc.). n= relative molecular mass/ Mr from emperical formula |
|
|
Compound X contains 40.0 % carbon, 6.6% hydrogen and 53.3% oxygen. Its relative molecular mass is 180. What is the molecular formula of X? |
Molecular mass of X = C6H12O6. |
|
|
What does Avogadro's law states about gases? |
Avogadro's Law states that equal volumes of all gases, under the same conditions and pressure, contain the same number of molecules. |
|
|
What is the molar volume of a gas? |
One mole of any gas occupies 24dm3 (24000 cm3) at room temperature (25°C) and pressure (1 atm). This volume is called the molar volume of a gas. |
|
|
How much volume does 2 mol of oxygen occupy? |
2 mol of volume occupy 2×24 = 48 dm3. |
|
|
How can we calculate the number of moles of a gas? State the formulae. (2) |
Calculating the number of moles of a gas: 1) Find the mass of the gas, The use the formula; number of moles of a gas = mass of gas in grams / Mr of gas. 2) Find the volume of the gas , Then use the formula; Number of moles of gas = volume of gas in cm3 at r.t.p / 24000 cm3. |
|
|
State the formula to find volume of a gas in cm3 and in dm3. |
Volume of gas (in cm3) = number of moles × 24000 Volume of gas (in dm3) = number of moles × 24 |
|
|
What is the volume, in dm3, of 8g of oxygen gas (O2) at r.t.p? |
Volume of oxygen = 6 dm3. |
|
|
In an experiment, hydrochloric acid was reacted with calcium carbonate at r.t.p , 80 cm3 of carbon dioxide was produced. Calculate the number of molecules of carbon dioxide given off. |
Number of molecules of carbon dioxide given off = 2.00 × 10^21 |
|
|
Calculate the mass of oxygen gas (O2) in a room that measures 4m high , 8m wide and 10m long. Assume that air contains 20% oxygen. (1 m3 = 10^6 cm3) |
Mass of oxygen = 8.53 × 10^4 g. |
|
|
Do balloons of the same mass contain the same number of particles? |
Balloons of the same mass doesn't contain the same number of particles. |
|
|
Explain an experiment to determine the emperical formula of copper(II) oxide. |
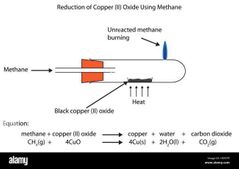
experiment to determine the emperical formula of copper(II) oxide: Procedure ; 1. Weigh a porcelain boat. 2. Put one spatula of copper(II) oxide (a black powder) in the boat and weigh again. 3. Put the porcelain boat containing copper(II) oxide into the middle of a Pyrex test tube with a small jet hole at its end. 4. Set up the apparatus as shown. 5.Allow methane gas (from the gas tap) to pass through the apparatus for about 30 seconds to remove air. 6. Light the gas that escapes through the hole in the test tube. Then heat the copper(II) oxide until no further colour change is observed. 7. The following reaction takes place during heating : 4CuO(s) + CH4(g) → 4Cu(s) +2H2O(g) + CO2(g) Copper will remain as a black residue in the porcelain boat. 8. Turn off the Bunsen Burner but allow the methane gas to flow through the apparatus while the apparatus is cooling. 9. Turn off the gas supply when the apparatus is cool. 10. Weigh the porcelain boat and its contents. Sample results; Mass of porcelain boat : 18.40 g Mass of porcelain boat + copper(II) oxide = 35.89 g Mass of porcelain boat + copper = 32.37 g Calculations: Mass of copper: 32.37 - 18.40 = 13.97 g Mass of oxygen: 35.89 - 32.37 = 3.52 g Number of moles ; Cu ; 13.97/ 64 = 0.22 = 0.22 Molar ratio : Number of moles ;Cu ; 13.97/ 64 = 0.22 O ; 3.52/ 64 = 0.22 Molar ratio : Cu : 0.22 / 0.22 = 10 : 0.22/0.22 = 1Thus the emperical formula of copper(II) oxide is CuO. Cu : 0.22 / 0.22 = 1 O ; 3.52/ 64 = 0.22 Molar ratio : Cu : 0.22 / 0.22 = 10 : 0.22/0.22 = 1Thus the emperical formula of copper(II) oxide is CuO. 0 : 0.22/0.22 = 1 Thus the emperical formula of copper(II) oxide is CuO. |

