![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
69 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Organic Chemistry |
Chemistry of compounds that contain the element C. |
|
|
|
Supernovae |
Massive explosion that scattered the elements in the universe. |
|
|
|
Vitalism |
Organic compounds were only those that came from living organisms and only living things could synthesize organic compounds through intervention of a vital force. Inorganic compounds were considered those compounds that came from non living sources. |
|
|
|
Friedrich Wöhler |
Discovered in 1828 that an organic compound called urea (a constituent of urine) could be made by evaporating an aqueous solution of the inorganic compound ammonium cyanate. |

|
|
|
Natural Products Chemistry |
Study of compounds from living organism |
|
|
|
Compounds |
Made up of elements combined in different proportions. |
|
|
|
Made up of atoms |
Molecules |
|
|
|
Atoms |
Consists of a dense positively charges nucleus containing protons and neutrons and a surrounding cloid of electrons. |
|
|
|
Protons and Neutrons have nearly equal masses and are about 1800 times heavy as Electrons. The volume of atom occupied by electron is 10,000 times larger than that of a nucleus. |
Note |
|
|
|
Atomic Number (Z) |
A number equal to the number of protons in its nucleus; also equals the number of electrons surrounding the nucleus. |
|
|
|
Isotopes |
Atoms of the same element that have different masses because they have different numbers of neutron. |
|
|
|
¹⁴C |
Radioactive; use for carbon dating. |
|
|
|
Deuterium atoms (²H) |
0.015% of the hydrogen atoms thatoccur naturally |
|
|
|
Tritium atoms ³H |
Radioactive hydrogen atom |
|
|
|
Valence Shell |
Outermost shell; electrons of this shell are the ones that an atom uses in making chemical bonds with other atoma to form compounds. |
|
|
|
Valence Electrons |
Equal to the group number of element. |
Carbon - 4 valence electrons. |
|
|
August Kekulé, Archibald Scott Couper & Alexander M. Butlerov |
Between 1858 to1861, working independently, laid the basis for the one of the most important theories in chemistry: The Structural Theory. |
|
|
|
Structural Theory |
1. Atoms in organic compounds can form a fixed number of bonds using their valence electrons. 2. A carbon atom can use one or more of its valence electrons to form bonds to other carbon atoms. |
|
|
|
Tetravalent |
Can form four bonds |
|
|
|
Divalent |
Can form two bonds |
|
|
|
Monovalent |
Can form one bond |
|
|
|
August Kekulé |
Gave the science of organic chemistry its modern definition: A study of the compounds of C atom. |
|
|
|
Isomers |
Different compound that have the same formula. |
|
|
|
Propylene Oxide |
Used with seaweed extract to make food grade thickeners |
|
|
|
Constitutional Isomers |
Different compounds that have the same molecular formula but differ in the sequence in which their atoms are bonded. |
|
|
|
J.H. Van't Hoff & J.a. Le Bel |
Working independently, proposed that the four bonds of the C atom in methane, for example, are arranged in such a way that they would point toward the corners of a tetrahedron, the carbon atom being placed at its center. |
|
|
|
G.N.Lewis & W. Kössel |
They first explain the nature of chemical bonds "Atoms without the electron configuration of a noble gas generally react to produce such a configuration because these configurations are known to be highly stable." |
|
|
|
Octet Rule |
The tendency for an atom to achieve a configuration where its valence shell contains eight electrons. |
|
|
|
Ionic Bonds (Electrovalent Bonds) |
Formed by the transfer of one or more electrons from one atom to another to create ions. Attractive force between oppositely charged ions. One source of such ions is a reaction between atoms of widely differing electronegativities. |
|
|
|
Electronegativity |
A measure of the ability of an atom to attract electrons. |
|
|
|
Ions |
Formed when an atom lose or gain electrons. |
|
|
|
Ionic Compounds (Salts) |
Form only when atom of very different electronegativities transfer electrons to become ions. |
|
|
|
Covalent Bonds |
Form bysharing of electrons between atoms of similar electronegativities to achieve the configuration of a noble gas. |
|
|
|
Molecules |
Are composed of atoms joined exclusively or predominantly by covalent bonds. |
|
|
|
Anion |
Negative ion |
|
|
|
Cation |
Positive Ion |
|
|
|
Formal Charge |
Formal Charge = no. of valence of electron - 1/2 (no.of shared electron) - no. of unshared electron. F = Z - 1/2S -U |
|
|
|
Resonance Theory |
States that whenever a molecule or ion can be represented by two or more lewis structure that differ only in the position of the electrons; 1. None of these structures which we call resonance structure or resonance contributors, will be realistic representation for the molecule or ion. None will be in complete accord with the physical or chemical properties of the substance. 2. The actual molecule or ion willl be better represented by a hybrid (average) of these structures. |
|
|
|
C-O single bond length |
1.43Å |
|
|
|
C-O double bond length |
1.20 Å |
|
|
|
1Å = _____ meters |
1×10^-10 |
|
|
|
The energy of the resonance hybrid is lower than the energy of any contributing structure. Resonance stabilizes a molecule or ion especially when resonance structures are equivalent. ( resonance stabilization) |
Note |
|
|
|
How to decide whether one resonance structure is more stable than another? |
1. The more covalent bonds a structure has, the more stable it is. 2. Charge separatio decreases stability. 3. Structure in which all the atoms have a complete valence shell of electrons are more stable. |
|
|
|
Erwin Schrödinger (Wave mechanics), Werner Heisenberg (Quantum Mechanics) & Paul Dirac |
A theory of atomic & molecular structure was advanced independently and almost simultaneously by three people in 1926: |
|
|
|
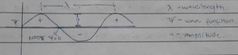
Wave function ψ |
Corresponds ro different energy state of electron. The relative probability of finding an.electron in a given region of space can be calculated from the wave function. |
|
|
|
Phase sign of a wave equation |
Indicates whether the solution is positive or nehative when calculated for a given point in space relative to the nucleus. |
|
|
|
Constructive interference |
Occurs when wave function with the same phase sign interact. There is a reinforcing effect and the amplitude of the wave increases. |
|
|
|
Louis de broglie |
First to put forth idea that electrons have properties of waves. |
|
|
|
Max Born |
Put forth the physical interpretation related to the electron wave function. |
|
|
|
Destructive interference |
Occurs when wave.functui with opposite phase signs interact. There is a subtractive effect and the amplitude of the wave goes to zero or changes sign. |
|
|
|
Squared of a wave function ψ² |
Expresses the probabilty of finding an electron at that location in space. Electron probability density is large if this is high. |
|
|
|
Orbital |
A region of space where the probability if finding an electron is high. |
|
|
|
Atomic Orbitals |
Are plots of ψ² in three dimensions. These plots generate the familiar s, p & d orbital shapes. |
|
|
|
Shape of s orbitals |
Sphere |
|
|
|
Shape of p Orbitals |
Lobes |
|
|
|
The + & - signs of wave functions do not imply positive or negative charge or greater or lesser probability of finding an electron. |
Note |
|
|
|
Relative energy of atomic orbitals 1s < 2s < 2p |
Note |
|
|
|
Degenerate orbitals |
Orbitals of equal energy (such as 3 2p orbitals) |
|
|
|
Aufbau Principle |
Orbitals are filled so that those of lowest energy are filled first. |
|
|
|
Aufbau Principle |
Orbitals are filled so that those of lowest energy are filled first. |
|
|
|
Pauli exclusion principle |
A maximum of 2 electrons may be placed in each orbital but only when the spins of the electrons are paired. |
|
|
|
Hund's Rule |
When we come to degenerate orbitals such as 3p orbitals, we add one electron to each with their spins unpaired until each of the degenerated orbitals contains one electron. Then we begin adding a second electron to each degenerate orbital so that the spins are paired. |
|
|
|
Heisenberg Uncertainty Principle |
We can not simultaneously know the position and momentum of an electron |
|
|
|
Molecular Orbital |
Represent the region of space where one or two electrons of a molecule are likely to be found. |
|
|
|
When Molecular Orbitals are formed by combinjng atomic orbitals, the number of molecular orbitals that result always equals the number of atomic orbitals that combine. |
Note |
|
|
|
Bonding Molecular Orbital ψmolec |
Results when two orbitals of the same phase overlap. High value of ψ & ψ². |
|
|
|
Antibonding Molecular Orbital ψ*molec |
Results when two orbitals of opposite phase overlap. ψ=ψ²=0 |
|
|
|
LCAO ( Linear Combination of Atomic Orbitals) |
Wave functions for the atomic orbitals are combined in a linear fashion (by addition or subtraction) in order to obtain new wave functions for the molecular orbitals. |
|
|

|

|
|

