![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
159 Cards in this Set
- Front
- Back
|
What four pathologies of the CV system can cause hypoperfusion?
|
1. Arteriosclerosis
2. Pump failure 3. Valvular disease 4. Arrhythmias |
|
|
Hypoperfusion of the coronary arteries can lead to what problems and/or symptoms?
|
Can cause:
Congestive heart failure Acute coronary syndromes Symptoms: Chest pain dyspnea Syncope Weakness Edema |
|
|
Hypoperfusion of the central nervous system can cause what kind of problems and/or symptoms?
|
Can cause transient, acute or chronic ischemic attacks.
Symptoms: Parathesias Weakness Dizziness Dysarthria Visual disturbances Syncope |
|
|
What kind of symptoms can hypoperfusion of the peripheral vasculature cause?
|
Can cause ischemia leading to claudication and erectile dysfunction
|
|
|
What are the 6 cardinal symptoms of cardiac disease?
|
1. Chest pain
2. Dyspnea 3. Edema 4. Fatigue 5. Syncope 6. Claudication |
|
|
What is chest pain caused by?
|
When oxygen supply to the myocardium is less than the metabolic need.
- Squeezing, heaviness, pressure, burning, aching. - Retrosternal radiating to neck, jaw, teeth, arms or shoulders. - Exacerbated by exertion, stress, heavy meals, and improved by rest. |
|
|
What helpful sign is sometimes present in patients with cardiac pain?
|

|
|
|
What are the definitions of dyspnea, orthopnea and paroxysmal nocturnal dyspnea?
|
Dyspnea is the “Subjective experience of breathing discomfort”
Orthopnea is SOB on recumbency. Paroxysmal nocturnal dyspnea is SOB on prolonged recumbency *Orthopnea and PND are symptoms of CHF *PND>> specific for CHF than orthopnea |
|
|
What is syncope?
|
Partial or complete loss of consciousness with interruption of awareness of oneself and ones surroundings.
Syncope is due to a temporary reduction in blood flow and therefore a shortage of oxygen to the brain. This leads to lightheadedness or a "black out" episode, a loss of consciousness. Temporary impairment of the blood supply to the brain can be caused by heart conditions and by conditions that do not directly involve the heart. |
|
|
What is claudication?
|
- Muscular pain/ache noted with exertion
- Usually located in the calf but can occur in any muscle - Pain occurs with exertion at a fixed distance and it is relieved by rest - Due to atherosclerosis impeding oxygen delivery to muscle tissue |
|
|
What are some signs of CV disease that can show with inspection of the patient?
|
1. Tachypnea
2. Cyanosis 3. Pallor 4. Clubbing 5. Edema 6. Jugular venous distention/pulsations 7. Carotid pulsations 8. Lifts/heaves |
|
|
What is cyanosis?
|
- Bluish discoloration of skin and mucous membranes
- Occurs when the reduced HGB concentration in capillary blood is below 4.0-5.0- g/dl - Can be central or peripheral |
|
|
What is clubbing?
|
- Increase longitudinal convexity of the nail plate
- Loss of unguophalangeal angle - Floating of the nail bed - Seen in multiple medical conditions; can be a sign of chronic hypoxia |
|
|
Image of clubbing of the fingernails.
|
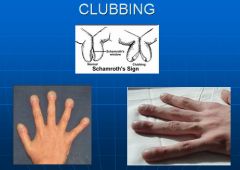
|
|
|
What is the cause of edema?
|
Loss of equilibrium between intravascular oncotic and hydrostatic pressures.
|
|
|
What is jugular venous distention?
|
- Occurs when central venous pressure > 8cm of water
- Measured by the top of the venous column in the neck with comparison to the sternal angle of Louis - The sternal angle of Louis is approximately 5-6 cm above the right atrium |
|
|
Picture of jugular venous distention:
|
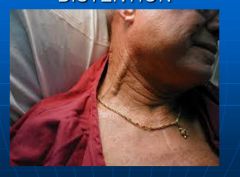
|
|
|
Why might percussion around the heart be useful?
|
To detect:
- Border of cardiac dullness - Pleural effusions - Abdominal aortic aneurysms |
|
|
X-ray image of a pleural effusion:
|
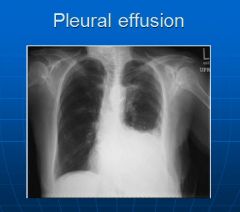
|
|
|
Picture of an AAA:
|
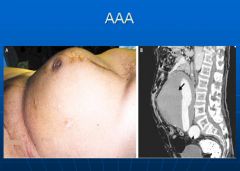
|
|
|
Flow of blood through the heart:
|
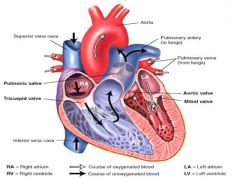
|
|
|
Anatomy of the coronary arteries:
|
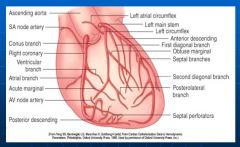
|
|
|
Placement of heart structures on normal x-ray:
|
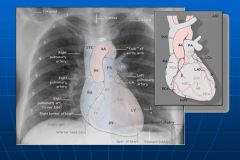
|
|
|
Normal chest x-ray side view:
|
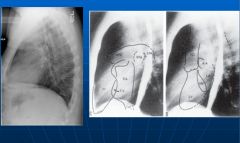
|
|
|
X-ray of mitral valve stenosis:
|
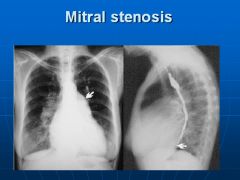
Note left ventricular enlargement
|
|
|
X-ray of mitral valve regurgitation:
|
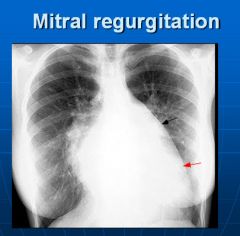
Left ventricle and left atrium enlargement; cannot see the aortic knob
|
|
|
X-ray of congestive heart failure:
|
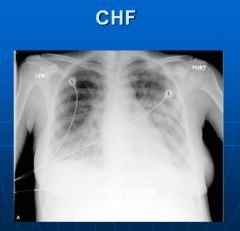
Venous engorgement, blunted costophrenic angle
|
|
|
Conduction system in the heart:
|
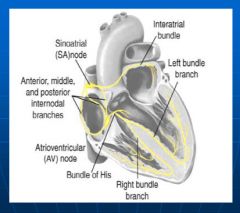
|
|
|
EKG waveforms and segments:
|
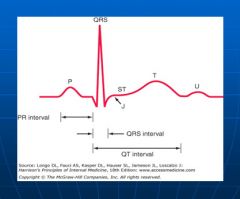
|
|
|
Testing Creatine Kinase for possible heart damage:
|
- Injury if CK-MB to
total CK > 2.5% Rise in 3-8 Hrs Peak at 24 Hrs Last 48-72 Hrs |
|
|
How long is troponin elevated for after heart damage?
|
Up to 6 days
|
|
|
What are some contraindications for a stress test?
|
- Resting angina within 48 hrs
- Unstable rhythm - Severe aortic stenosis - Acute myocarditis - Uncontrolled heart failure - Severe pulmonary hypertension - Active infective endocarditis |
|
|
What test is the "gold standard" for detecting valve abnormalities?
|
2-dimensional echocardiogram
|
|
|
How does Doppler Echocardiography work?
|
- Uses ultrasound reflecting off RBC’s to measure the velocity of blood flow
- Velocities are superimposed on a 2D echocardiographic image - Color flow is used to measure blood velocity on real time. Red is toward the transducer and blue is away from the transducer. Green reveals turbulence |
|
|
What is the Doppler Echo good for?
|
- Measure valve gradients
- Quantitate valvular regurgitation - Measure intracardiac pressures - Measure cardiac output - Evaluate diastolic filling - Asses congenital heart disease |
|
|
Nuclear cardiology imaging:
|
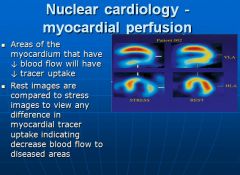
|
|
|
Normal blood pressures in the cardiovascular circulation:
|
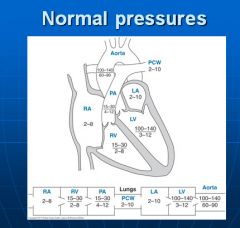
|
|
|
What are the 3 layers of vessel walls?
|
From inside out; intima, media and adventitia.
|
|
|
What are the 3 different types of arteries?
|
1. Large, or elastic arteries.
2. Medium-sized, or muscular arteries. 3. Small arteries and arterioles. * Arterioles are the principle control points for regulation of resistance to blood flow. This modulation is accomplished by changes in lumen size. *Resistance to blood flow is inversely proportional to the fourth power of the diameter; halving the diameter increases resistance 16-fold. |
|
|
What type of response occurs when vessels are damaged?
|
Vascular injury with endothelial cell loss or even merely dysfunction, stimulates smooth muscle cell growth and associated matrix synthesis. The SMC migrate to the intima to perform these functions.
Thus, intimal thickening is essentially the response of the vessel wall to any insult. With persistant or reccurent insults, excessive thickening can cause stenosis or small and medium sized blood vessels that impede downstreatm tissue perfusion. |
|
|
What is arteriolosclerosis?
|
Affects small arteries and arterioles. Two anatomic variants, hyaline and hyperplastic, are both associated with vessel wall thickening. Arteriolosclerosis is most often associated with hypertension and/or diabetes mellitus.
|
|
|
What is atherosclerosis?
|
Characterized by intimal lesions called atheromas that protrude into vascular lumen. An atheromatous plaque consists of a raised lesion with a soft, yellow, grumous core of lipid covered by a firm, white fibrous cap.
|
|
|
What are the 3 major constitutional risk factors for Ischemic Heart Disease?
|
1. Age: incidence increases with age
2. Gender: men more susceptible at an earlier age - postmenopausal women have greater risk. 3. Genetics: predisposition to hypertension, diabetes or dyslipidemia |
|
|
What are the 4 major modifiable risk factors for Ischemic Heart Disease?
|
1. Hyperlipidemia: Increased cholesterol and high LDL are a major risk factor even in the absence of other risk factors.
2. Hypertension: Untreated hypertension can increase the risk of IHD by 60% 3. Smoking: 4. Diabetes: induces hypercholesterolemia. Incidence of MI is twice as high in diabetic individuals. 100 fold increased risk of atherosclerosis induced gangrene of the lower extremities. |
|
|
What are the 3 other factors that may play a role in increased risk of IHD?
|
1. Inflammation as marked by CRP
2. Hyperhomocysteinemia 3. Increased Lipoprotein a: An altered form of LDL that contains the apolipoprotein B-100 portion of LDL linked to apoA |
|
|
Describe the "response-to-injury" hypothesis of atherosclerosis:
|
This model views atherosclerosis as a chronic inflammatory response of the arterial wall to endothelial injury. Lesion progression occurs through interactions of modified lipoproteins, monocyte derived macrophages, T lymphocytes and the normal cellular constituents of the arterial wall.
|
|
|
What are the steps involved in the pathogenesis of atherosclerosis according to the "response to injury" hypothesis:
|
1. Chronic endothelial injury, with resultant endothelial dysfunction, causing increased permeability, leukocyte adhesion and thrombosis.
2. Accumulation of lipoproteins (mainly oxidized LDL) in the vessel wall. 3. Monocyte adhesion to the endothelium followed by migration into the intima and transformation into macrophages and foam cells. 4. Platelet adhesion. 5. Factor release from activated platelets, macrophages and vascular wall cells inducing SMC recruitment, either from the media or from circulating precursors. 6. SMC proliferation and ECM production. 7. Lipid accumulation both extracellularly and within cells. |
|
|
What are the 3 most important contributors to vessel endothelial cell dysfunction?
|
1. Hemodynamic disturbance: plaques tend to occur at juctions of exit points, branch points and along the posterior wall of the abdominal aorta.
2. Hypercholesterolemia: - High cholesterol can impair endothelial cell function by increasing production of reactive oxygen species, leading to accelerated decay of NO, which leads to vasoconstriction and local shear stress. - Lipids are oxidized via oxygen free radicals generated by macrophages or EC's. Oxidized LDL is ingested by macrophages through scavenger receptors resulting in foam cell formation. Oxidized LDL also stimulates release of factors that increase monocyte recruitment into lesions. 3. Inflammation: - Early in atherogenesis dysfunctional EC's express adhesion molecules that encourage leukocyte adhesion; VCAM-1 in particular binds monocytes and T cells. - Macrophage activation via oxidized LDL or T cells result in cytokine production that further increases leukocyte adhesion and chemokine production that in turn propel mononuclear inflammatory cell recruitment. - T cells recruited to the intima interact with macrophages to produce a chronic inflammatory state. - As a consequence of the chronic inflammatory state, activated leukocytes and vascular wall cells release growth factors that promote SMC proliferation and ECM synthesis. |
|
|
What are the most common sites of development of atherosclerotic plaques?
|
In descending order:
Lower abdominal aorta, coronary arteries, popliteal arteries, internal carotid arteries and vessels of the circle of Willis. |
|
|
What are atherosclerotic plaques composed of?
|
Typically, the fibrous cap is composed of SMC's and dense collagen.
Beneath and to the sides is a more cellular area containing macrophages, T cells and SMC's. Deep to the fibrous cap is a necrotic core containing lipid, debris, foam cells, fibrin, cholesterol crystals and thrombi. |
|
|
What 4 pathological changes are atherosclerotic plaques susceptible to?
|
1. Rupture, ulceration or erosion: Can lead to thrombi formation with partial or complete occlusion of the vessel leading to downstream ischemia.
2. Hemmorhage into a plaque: a contained hematoma may expand the plaque or induce rupture. 3. Atheroembolism: plaque rupture can discharge debris into the bloodstream, producing microemboli composed of plaque contents. 4. Aneurysm formation: Plaque induced pressure or ischemic atrophy of the underlying media, with loss of elastic tissue, causes weakness of the vessel wall and development of aneurysms that may rupture. |
|
|
What is a "true" aneurysm?
|
A localized abnormal dilation of a blood vessel or the heart that involves all three layers of the arterial wall.
* Atherosclerotic, syphilitic, congenital and ventricular aneurysms that follow transmural myocardial infarctions are of this type. |
|
|
Naming of different types of aneurysms:
|
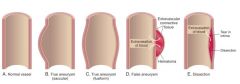
|
|
|
What are the 2 most important causes of aortic aneurysms?
|
1. Atherosclerosis
2. Cystic medial degeneration of the arterial media |
|
|
Pathogenesis of Abdominal Aortic Aneurysm:
|
1. AAA occurs more frequently in men and rarely before age 50.
2. Atherosclerosis is a major cause of AAA. 3. AAA results from an altered balance of collagen degradation and synthesis mediated by local inflammatory infiltrates and the destructive proteolytic enzymes they produce. 4. Matrix metalloproteinases have been increasingly implicated in AAA development; these enzymes have the ability to degrade all components of the ECM in the arterial wall. 5. Decreased levels of tissue inhibitor of metalloproteinases also contribute to overall ECM degradation. |
|
|
Clinical consequences of an AAA:
|
1. Rupture into the peritoneal cavity or retroperitoneal tissues with massive, potentially fatal hemorrhage.
2. Obstruction of a branch vessel resulting in downstream tissue ischemic injury. 3. Embolism from atheroma or mural thrombus. 4. Impingement on an adjascent structure. 5. Presentation as an abdominal mass that simulates a tumor. |
|
|
How is AAA size related to risk of rupture?
|
AAA less than 4 cm have a zero change of rupture.
For every increase in size beyond that there is an increase in the chance of rupture. |
|
|
How does tertiary syphilis cause AAA?
|
The organism infects the vasa vasorum of the aorta, leading to obliterative endarteritis; a narrowing of the lumen and scarring of the vessel wall. Will also see a dense surrounding rim of plasma cells that may extend into the media.
The narrowing of the vasa vasorum leads to ischemic injury of the aortic media. With destruction of the media, the aorta loses its elastic recoil and may become dilated, producing an aneurysm. * These aneurysms tend to be thoracic instead of abdominal; can lead to heart damage. |
|
|
What is an aortic dissection?
|
A catastrophic event whereby blood splays apart the laminar planes of the media to form a blood filled channel within the aortic wall; this channel often ruptures through the adventitia and into various spaces, where it either causes massive hemorrhage or cardiac tamponade (hemorrhage into the pericardial sac).
|
|
|
Epidemiology of aortic dissection:
|
Occurs most often in:
1. Men aged 40-60 years with pre-existing hypertension. 2. Younger patients with systemic or localized abnormalities of connective tissue (Marfans syndrome) * Can also rarely happen during or after pregnancy |
|
|
Pathogenesis of an aortic dissection:
|
1. Hypertension is THE major risk factor for dissection.
2. Inherited or acquired risk factors are connective tissue disorders such as Marfans, Ehlers-Danlos, vitamin C deficiency and copper metabolism defects. |
|
|
What type of lesion most frequently precedes the development of a dissection?
|
Cystic medial degeneration:
CMD is characterized by elastic tissue fragmentation and separation of the elastic and SMC elements of the media by cystic spaces filled with the amorphous proteoglycan-rich ECM. |
|
|
How are dissections classified?
|
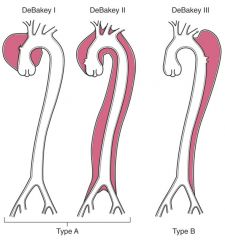
Type A: more common and more dangerous type; involve either the ascending aorta only, or both the ascending and descending aorta.
Type B: Distal lesions not involving the ascending part and usually beginning distal to the subclavian artery. |
|
|
What are the symptoms and clinical manifestations of a dissection?
|
Symptoms:
Sudden onset of excrutiating pain, usually beginning in the anterior chest, radiating to the back between the scapula and moving downward as the dissection progresses. Clinical manifestations: Cardiac tamponade, aortic insufficiency, myocardial infarction or extension of the dissection into the great arteries of the neck or into the coronary, renal, mesenteric or iliac arteries, causing critical vascular obstruction. * The most common cause of death is rupture of the dissection outward into any of the three body cavities; pericardial, pleural and peritoneal. |
|
|
What kinds of antithrombic molecules are produced by vessel endothelium?
|
Heparan sulfate
Thrombomodulin Plasminogen activators Prostacyclin Nitric Oxide |
|
|
What 2 vasoactive and inflammatory mediators are synthesized by vessel smooth muscle cells?
|
IL-6 and TNF-a; both promote leukocyte proliferation and induce endothelial expression of leukocyte adhesion molecules (LAM).
* These synthetic functions become more prominent at sites of atherosclerotic plaque and may contribute to their pathogenesis. |
|
|
Diagram of the formation of foam cells:
|
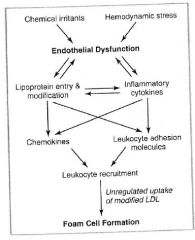
Endothelial cells and foam cells play a central role in formation of the fatty streak, but smooth muscle cell migration into the intima dominates early plaque progression.
|
|
|
Lipoprotein transport system:
|
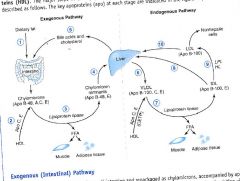
|
|
|
Role of the foam cell in fibrous plaque formation:
|
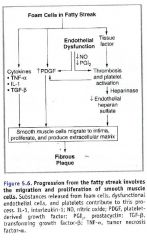
|
|
|
Describe the way that fibrous cap formation and degredation are balanced:
|
1. Smooth muscle cells secrete collagen that fortify the cap; stimulated by PDGF and TGF-b.
2. T cells secrete INF-g; which inhibits smooth muscle collagen synthesis. 3. Local foam cells are stimulated by inflammatory cytokines to produce MMP; MMP degrades collagen and elastin. |
|
|
Diagram of fibrous cap remodeling:
|
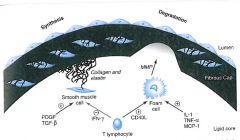
|
|
|
What is the term for intermittent chest discomfort on exertion?
|
Angina pectoris
|
|
|
What is the defect in familial hypercholesterolemia?
|
A genetic defect in the LDL receptor; patients with this defect cannot remove LDL from the circulation efficiently
|
|
|
What receptor does polyunsaturated fat activate?
|
The PPAR-a receptor; leads to increased HDL apoprotein (Apo1a) and lipoprotein lipase.
|
|
|
What are the names for a hardening of the arterioles,
muscular arteries and elastic arteries? Atherosclerosis |
Arteriolosclerosis
Monckeberg medial sclerosis Atherosclerosis |
|
|
Histology of arteriolosclerosis:
|
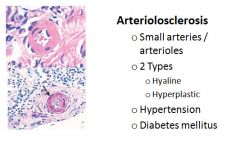
|
|
|
Histology of Monckeburg medial sclerosis:
|
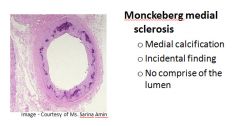
|
|
|
Diagram of endothelial cell dual role to inhibit and promote thrombogenesis:
|
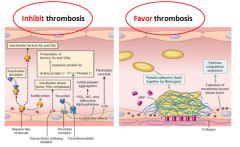
|
|
|
Histology of foam cells:
|
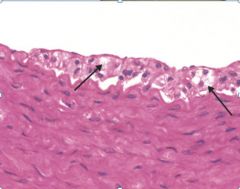
|
|
|
Histology of a stable vs an unstable plaque:
|
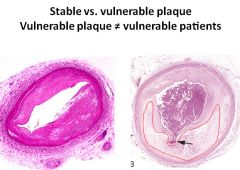
Arrow is pointing to a rupture.
Area outlined in red is the fatty plaque within the vessel. |
|
|
Possible physical findings on a patient with atherosclerosis related problems:
|
Loss of pulses distal to obstruction
Bruits over disease arteries Loss of hair Muscle atrophy Pallor Cyanosis Necrosis/gangrene Decrease temperature Rubor Delayed capillary refill |
|
|
What is the ankle-brachial index?
|
Comparison of systolic brachial artery pressure to posterior tibial artery pressure
Normal> .9 .75-.9= mild disease .6-.75= moderate disease < .6 severe .5 or less = limb threatening |
|
|
Possible consequences of a thoracic aortic aneurysm with progression:
|
Carotid occlusion= Cerebral ischemia- CVA
Spinal arteries= paraplegia and anesthesia Subclavian= limb ischemia and B/P difference between the upper extremities Renal artery occlusion= infarction and renal failure |
|
|
What is the pharmacological treatment for a TAA?
|
B-blockers and vasodilators
|
|
|
What are some possible physical exam findings on a patient with an AAA?
|
High heart rate
Low blood pressure Distended abdomen Abdominal bruit Pulsatile mass Cool lower extremities Decreased pulses in lower extremities Livedo reticularis in lower extremities. |
|
|
Livedo Reticularis:
|
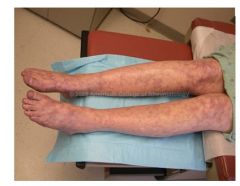
|
|
|
Epidemiology, risk factors and progression of AAA:
|
Most due to atherosclerosis in the elderly
Males > females Risk factors: smoking , HTN and dyslipidemia Usually asymptomatic until significant enlargement occurs or there is quick progression of disease Dissection presents with severe abdominal, flank or back pain. Hypotension and tachycardia are common signs Signs of tissue ischemia below the dissection can be present |
|
|
MRI of an AAA:
|
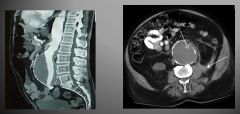
|
|
|
What are the four types of hypersensitivity reactions?
|
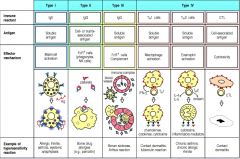
|
|
|
Vasculitis overview table:
|

|
|
|
What is the mechanism for ANCA mediated vasculitis?
|
1. Neutrophil release of PR3 or MPO incites ANCA formation in a susceptible host.
2. Some underlying disorder (infection, endotoxin exposure) elicits inflammatory cytokines, such as TNF, that result in surface expression of PR3 and MPO on neutrophils and other cell types. 3. ANCA's react with these cytokine-primed cells and either cause direct injury (to endothelium) or induce activation (in neutrophils) 4. ANCA activated neutrophils degranulate and also cause injury by the release of reactive oxygen species, engendering EC toxicity and other direct tissue injury. |
|
|
What are the 3 immunological mechanisms that initiate non-infectious vasculitis?
|
1. Immune complex-associated vascultitis
2. Antineutrophil Cytoplasmic Antibodies 3. Anti-Endothelial Cell Antibodies |
|
|
Giant Cell (Temporal) Arteritis:
|
- The most common of the vacultitides.
- Chronic, granulomatous inflammation of large to small-sized arteries. - Principally effects the arteries in the head, but also the vertebral, opthalmic and aorta. - Caused by a T-cell mediated immune response. - Occurs in persons over 50 and more often in women. - Classic symptom is intense pain over the superficial temporal artery. - Ocular symptoms abruptly appear in 50%; may lead to diplopia or complete vision loss. - Increased sedimentation rate and CRP - Diagnose with a biopsy. - Treat with corticosteroids. |
|
|
Takayasu Arteritis:
|
- A granlomatous vasculitis of medium and larger sized arteries.
- Characterized by ocular disturbances and weakening of the pulses in the upper extremities. - Manifests with fibrous thickening of the aortic arch and great vessels, with severe lumen narrowing of the major branch vessels. - Occurs most frequently in women younger than 40 of asian descent. - Dominant symptoms are reduced blood pressure and weaker pulses in the upper extremities than the lower. Coldness or numbness of the fingers, ocular disturbances, retinal hemorrhage, blindness or neurological deficits - Labs show anemia, increased ESR and increased Ig's - Treat with corticosteroids and/or angioplasty for effected vessels. |
|
|
Polyarteritis Nodosa:
|
- A systemic vasculitis of small or medium sized muscular arteries; typically involving renal and visceral vessels.
- Vessels of the kidneys, heart, liver and GI tracts are involved in descending order of frequency, usually at branch points. - Usually affects young adults. - Symptoms are malaise, fever, weight loss, rapidly developing hypertension, abdominal pain, melena, myalgia, peripheral neuritis - Biopsy often needed to confirm diagnosis - Immune complex mediated - 30% of patients have hepB antigenemia and HBsAg-HBsAb immune complexes can be found. - Can cause aneurysms - Treat with corticosteroids and cyclophosphamide |
|
|
Kawasaki Disease:
|
- An acute febrile, usually self-limited illness of infancy or childhood (most are younger than 4 years) associated with an arteritis affecting large to medium-sized and even small vessels
- Can involve the coronary arteries; this can result in aneurysms that rupture or thrombose, causing acute MI. * Leading cause of acquired heart disease in children - Though to arise from a delayed-type hypersensitivity response of T cells to an unknown vascular antigen. This leads to cytokine production and B cell activation, with formation of autoantibodies to EC's and SMC's. - Presents with conjuctival and oral erythema and erosion, edema of the hands and feet, erythema of the palms and soles, a desquamative rash, and cervical lymph node enlargement. - Treat with IV immunoglobulin |
|
|
Microscopic Polyangiitis:
|
- A necrotizing vasculitis that generally affects capillaries as well as arterioles and small venules.
- The skin, mucous membranes, lungs, brain, heart, GI tract, kidneys and muscle can all be involved; necrotizing glomerulonephritis and pulmonary capillaritis are common. - An antibody response to antigens such as drugs, microorganisms, proteins or tumor products is the cause. - This results in immune complex deposition, or can trigger secondary immune responses. - p-ANCA's usually present - Major features include hemoptysis, hematuria and proteinuria; bowel pain or bleeding, muscle pain or weakness and palpable cutaneous purpura. - Treatment is the removal of the offending agent. |
|
|
Wegener Granulomatosis:
|
- A necrotizing vasculitis characterized by a triad of:
Acute necrotizing granulomas of the upper or lower respiratory tract. Necrotizing or granulomatous vasculitis affecting small to medium sized vessels; most prominent in the lungs and upper airways. Renal disease in the form of focal necrotizing, often crescentic glomerulonephritis. - Probably caused by a cell-mediated hypersensitivity response to an inhaled infectious or environmental agent. - c-ANCA's usually present along with anemia and increased ESR. - Males affected more often that females at an age of about 40 years. Mostly whites affected. - Classic features are persistent pneumonitis with bilateral nodular and cavitary infiltrates, chronic sinusitis, mucosal ulcerations of the nasopharynx and evidence of renal disease. - Biopsy first to r/o infectious granulomatous disease. - Treat with cyclophosphamide. |
|
|
Thromboangiitis Obliterans (Buerger Disease):
|
- A disease that often leads to vascular insufficiency characterized by segmental, thrombosing acute and chronic inflammation of medium-sized and small arteries
- Principally occurs in the tibial and radial arteries - Occurs in heavy smokers, usually before age 35. - Though to involve direct toxicity to endothelium by tobacco products - Early signs are nodular phlebitis, cold sensitivity of the Reynaud type in the hands and pain in the instep of the foot induced by exercise. - Vascular insufficiency accompanied by severe pain, even at rest. - Chronic ulcerations of the toes, fingers and feet may be follwed by gangrene. - Treatment is abstinence from smoking. |
|
|
Infectious Vasculitis:
|
- Usually caused by Aspergillus or Mucor species.
- Vascular infections can weaken arterial walls and cause mycotic aneurysms, or they can induce thrombosis and infarction. - |
|
|
Raynaud Phenomenon:
|
Results from an exaggerated vasoconstriction of digital arteries and arterioles.
Primary: occurs mostly in young women in response to cold or emotion. Usually benign. Secondary: Vascular insufficiency of the extremities in the context of underlying disease. Any patient with symptoms should be evaluated; 10% will eventually manifest their underlying disease. * Color change goes from white to blue to red. |
|
|
Varicose Veins:
|
- Are abnormally dilated, tortuous veins produced by prolonged increase in intramural pressure and loss of vessel wall support.
- The superficial veins of the upper and lower leg are typically involved. - Varicose dilation renders the venous valves incompetent and leads to stasis, congestion, edema, pain and thrombosis. - Disabling sequelae include persistant edema in the extremity and secondary ischemic skin changes including stasis dermatitis and ulcerations; poor wound healing and superimposed infections can become chronic varicose ulcers. - Esophageal varices: Liver cirrhosis causes portal vein hypertension. This leads to increased blood flow at the gastroesophageal junction, the rectum (hemorrhoids) and periumbilical veins of the abdominal wall. Esophageal varices are the most important, since their rupture can lead to massive upper GI hemorrhage. |
|
|
Thrombophlebitis or phlebothrombosis:
|
- The deep leg veins account for more than 90% of all cases.
- Congestive heart failure, neoplasia, pregnancy, obesity, the postoperative state, and prolonged immobilization are the most important clinical predispositions. - In patients with cancer, venous thrombosis classically appear in one site, disappear and then reoccur in other veins (Trousseau sign) - Local manifestations include distal edema, cyanosis, vein dilation, heat, tenderness, redness, swelling and pain; but may be absent. - Pain may elicited by pressure of squeezing of the area involved, or by dorsiflexion of the foot (Homan sign). - Pulmonary embolism is a common and serious clinical complication of DVT. |
|
|
Superior and Inferior Vena Cava syndromes:
|
- Superior vena cava syndrome is usually caused by neoplasms that compress or invade the superior vena cava. The resulting obstruction produces marked dilation of the veins of the head, neck and arms with cyanosis. Pulmonary vessels can also become compressed, inducing respiratory distress.
- Inferior vena cava syndrome can be caused by neoplasms that compress or invade the inferior vena cava or by a thrombus from the hepatic, renal or lower extremity veins that goes upwards. Obstruction induces marked lower extremity edema, distention of the superficial collateral veins of the lower abdomen, and - with renal vein involvement - massive proteinuria |
|
|
Lymphangitis and Lymphedema:
|
- Lymphangitis is the acute inflammation elicited when bacterial infections spread into and through the lymph system; the most common agents are group A B-hemolytic strep. Lymphangitis is recognized by red, painful subcutaneous streaks with painful enlargement of the draining lymph nodes.
- Primary lymphedema can occur as a congential defect resulting from lymphatic agenesis or hypoplasia. Secondary lymphedema represents the accumulation of fluid behind a blockage of previously normal lymphatics; tumors, surgical removal, postirradiation fibrosis, filariasis, postinflammatory thrombosis or scarring |
|
|
Pathological outcomes of vasculitis:
|
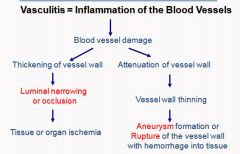
Vasculitis is a clinicopathologic process characterized by inflammation of and damage to blood vessels. The vessel lumen is usually compromised, and this is associated with ischemia of the tissues supplied by the involved or the vessel wall may weaken as a result of inflammation resulting in rupture of the vessel wall with hemorrhage in the affected tissue/organ or if chronic may lead to aneurysmal dilation..
|
|
|
Vessel sizes in Vasculitis
|
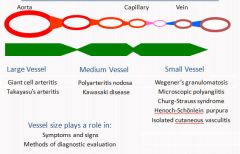
Vasculitidies pose multiple challenges. Most vasculitis entities are syndromes with great degree of heterogeneity and overlap of clinical presentation. Second pathogenesis of many syndromes is not well established. One option is to classify the vasculitidies based on the size of the affected vessels. Vessel type and size determines the clinical presentation and application of various diagnostic tests or procedures.
|
|
|
Vasculitis classficiation list:
|
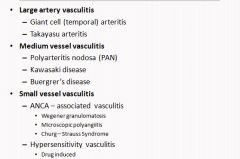
The vasculitidies are aggregated into 3 groups based on the size of the vessel affected. Giant cell arteritis and Takasyasu diseases may share common pathogenetic mechanism and size of vessels involved. However, the 3 diseases grouped in the medium size vessel category are of diverse pathogenesis. Similarly the 2 disease groups in the small size vessel groups have different and yet ill defined pathogenetic mechanisms.
|
|
|
p-ANCA and c-ANCA staining:
|
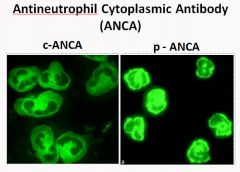
|
|
|
Generalized symptoms of vasculitis:
|

|
|
|
Organ specific manifestations of vasculitis:
|
- Skin
Purpura, nodules, ulcers, gangrene - Peripheral nervous system Mononeuritis, polyneuropathy - Central nervous system Stroke, seizure, - Kidney Hypertension, proteinuria, hematuria, renal failure - Heart Infarction, cardiomyopathy, pericarditis, arrhythmia - Lung Cough, chest pain, hemoptysis, dyspnea - Eyes Blindness, scleritis - Gastrointestinal tract Pain, bleeding, perforation |
|
|
Common lab findings in vasculitis:
|

|
|
|
Vasculitis treatment algorithm:
|
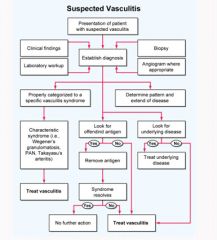
|
|
|
Histology of Giant Cell Arteritis:
|
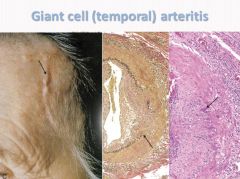
|
|
|
Histology of Takayasu Arteritis:
|
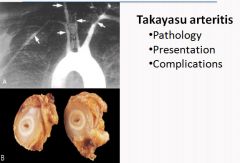
|
|
|
Histology of Takayasu Arteritis:
|
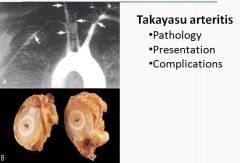
|
|
|
Histology of Wegener's Granulomatous Vasculitis:
|
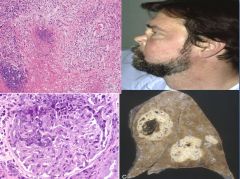
“Saddle Nose” is an example of ischemia of small arteries contributing to cartilage and bone loss.
Granuloma formation is a result of type IV immune reaction. Capillary reaction is a type III. |
|
|
Histology of polyarteritis nodosa:
|
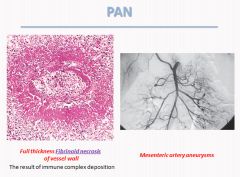
For this diagnosis, clinical and laboratory findings should be consistent and the diagnosis could be confirmed by biopsy of target organ e.g. skin or muscle biopsy or by angiography of the affected circulations. At times a random biopsy of small artery may be of diagnostic value.
|
|
|
Photos of Kawasaki Disease:
|
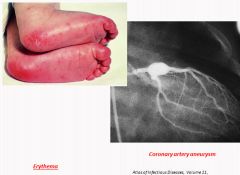
|
|
|
Veins of the lower extremities:
|
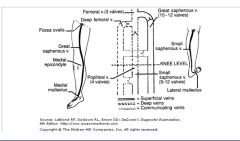
|
|
|
What is Virchow's Triad?
|
1: Abnormal blood flow
2: Hypercoagulability 3: Endothelial injury * Applicable to disorders of coagulation or thrombosis |
|
|
Diagnosis of a Deep Vein Thrombosis:
|

|
|
|
Treatment of a DVT:
|

|
|
|
Images of conditions caused by chronic venous insufficiency:
|
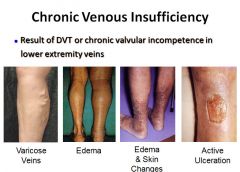
|
|
|
Image of subclavian vein thrombosis:
|
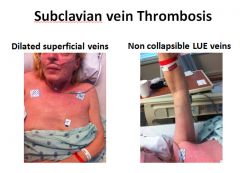
Horner's syndrome (also Horner syndrome is the combination of drooping of the eyelid (ptosis) and constriction of the pupil (miosis), sometimes accompanied by decreased sweating of the face on the same side.
Horner's syndrome can be seen in subclavian vein thrombosis due to compression of one side of the cervical or thoracic sympathetic chain, which generates symptoms on the ipsilateral (same side as lesion) side of the body |
|
|
Review of the lymphatic system:
|
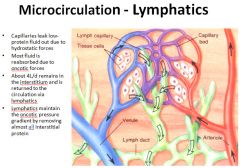
|
|
|
Presentation of edema in lymphedema:
|
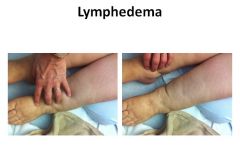
|
|
|
Presentation of edema with venous problems:
|
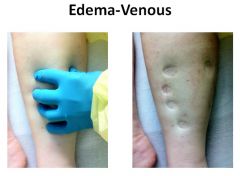
|
|
|
What is it called when a patient has hypertension with no discernable cause?
|
Essential hypertension
|
|
|
At what systolic and diastolic blood pressures is hypertension diagnosed?
|
Over 140 mmHg systolic
Over 90 mmHg diastolic * Systolic pressure is more important than diastolic in determining cardiovascular risk |
|
|
What two variables by are important in determining blood pressure?
|
1. Cardiac output: affected by blood volume; strongly dependant on sodium concentrations.
2. Peripheral vascular resistance: regulated at the level of arterioles and influenced by neural and hormonal inputs. Normal vascular tone reflects an interplay between vasoconstriction and vasodilation. Resistance vessels also exhibit autoregulation, whereby increased flow induces constriction to protect tissues against hyperperfusion. Other local factors, such as pH and hypoxia are also involved. |
|
|
Which two organs are the key players in blood pressure regulation?
|
1. Kidneys:
Renin produced by the juxtaglomerular cells is released in response to decreased perfusion to the afferent arterioles. Plasma angiotensinogen is converted to angiotensin I by renin; further converted to angiotensin II by ACE in the lungs. Angiotenin II induces vascular SMC contraction and stimulates aldosterone secretion in the adrenals. 2. Adrenals |
|
|
What is "malignant" hypertension?
|
A rapidly rising blood pressure that if untreated leads to death within 1-2 years.
Characterized by severe hypertension (diastolic over 120 mmHg), renal failure, and retinal hemorrhages and exudates, with or without papilledema. |
|
|
What is likely a key initiating factor for hypertension?
|
Reduced renal excretion of sodium.
- Decreased Na excretion will cause a rise in fluid volume and increased cardiac output, thereby elevating BP. At the higher setting of BP, enough additional Na will be excreted by the kidneys to equal intake and prevent fluid retention. Thus a new steady state of Na excretion would be achieved, but at the expense of elevated BP. |
|
|
What are the pathological outcomes of hypertension?
|
1. Accelerated atherosclerosis
2. Aortic dissection 3. Cerebrovascular hemorrhage 4. Hyaline arteriolosclerosis 5. Hyperplastic arteriolosclerosis |
|
|
Hyaline arteriolosclerosis:
|
Homogenous pink hyaline thickening of the walls of arterioles with narrowing of hte lumen.
Caused by leakage of plasma proteins across the ECM and excessive ECM production by SMC's in response to the hemodynamic stress of hypertension. |
|
|
Hyperplastic arteriolosclerosis:
|
Characteristic of malignant hypertension.
Associated with "onion-skin", concentric, laminated thickening of the arteriole walls with luminal narrowing. The rings are composed of SMC's and thickened basement membrane. |
|
|
Demographics of hypertension:
|
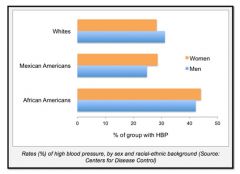
|
|
|
Graph of changes in blood pressure:
|
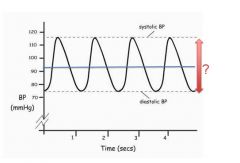
SBP is the highest BP measured in the arterial circulation at any point in the cardiac cycle.
DBP is the lowest pressure measured in the systemic circulation at any point in the cardiac cycle. The line is the mean arterial pressure. (mostly from diastoly because we spend 2/3 of the time in diastoly) |
|
|
Blood pressure equations part 1:
|
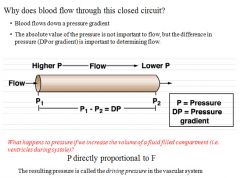
|
|
|
Blood pressure equations part 2:
|

|
|
|
Blood pressure equations part 3:
|
BP = CO x TPR
CI = SV x HR |
|
|
What is syncope?
|
A vasovagal reaction: stress relaxation of vessels leading to decreased return of blood to the heart --> decreased cerebral blood flow.
|
|
|
"Set point" regulation of blood pressure:
|
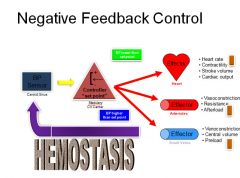
|
|
|
Determinants of blood pressure:
|
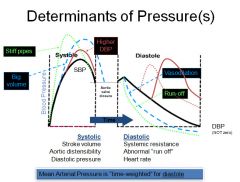
|
|
|
Role of sodium in BP:
|

|
|
|
Eutrophic remodeling:
|

Eutrophic remodeling means the tissue is rearrangement of the same tissue that was already there…not hypertrophy.
This is what happens when the vessel decides that there should be no additional blood coming into the area. |
|
|
Blood pressure range classification:
|
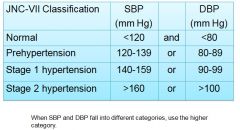
US Preventive Services Task Force recommendations are to establish diagnosis only after:
2 or more elevated readings On at least 2 visits Over a period of at least 1 week |
|
|
Blood pressure range classification:
|
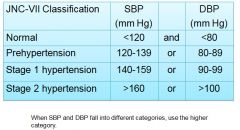
US Preventive Services Task Force recommendations are to establish diagnosis only after:
2 or more elevated readings On at least 2 visits Over a period of at least 1 week |
|
|
Facts about pre-hypertension:
|
- Is not a disease
- Is not an indication for pharmacological treatment - Does not have a BP goal, - Does predict a higher risk for developing CV events, - Does predict a higher risk for developing HTN, - Should be an incentive to improve lifestyle practices for prevention of HTN and CVD. - Recheck in about one year |
|
|
What 3 things should be evaluated in the patient with hypertension?
|
1. Assess lifestyle and identify other CV risk factors or concomitant disorders that affects prognosis and guides treatment.
2. Reveal identifiable causes of high BP. 3. Assess the presence or absence of target organ damage and CVD. |
|
|
Obesity induced hypertension:
|
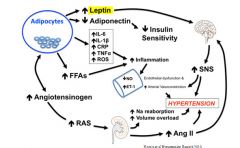
|
|
|
What role does genetics play in the development of hypertension?
|
- Genetics
Hypertension clusters in families Multiple genes are involved Identifiable single gene of hypertension is not common- (Liddle’s syndrome) * If the parents develop hypertension at the age of 55 or earlier, the lifetime risk for the children is seven-fold higher than normal. |
|
|
Complications of Hypertension:
|
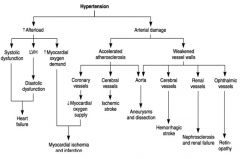
|
|
|
Effect of lifestyle modification on blood pressure:
|
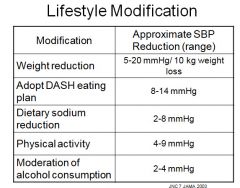
Weight reduction + DASH is equivalent to 1-2 antihypertensives
|
|
|
How do antihypertensive drugs work?
|
- All antihypertensive drugs act on the familiar formula:
BP = SVR x CO (HR v SV) They act by: Reducing SVR or by... Reducing cardiac output … or by… Reducing heart rate … or by… Reducing stroke volume |
|
|
What are the blood pressure goals for normal vs high risk patients?
|
General population: BP <140/90 mmHg based on worldwide standards
High-risk groups (diabetes or chronic kidney disease): BP <130/80 mmHg based on increased absolute risk of CVD in these groups |
|
|
Patients who should be evaluated for secondary hypertension:
|
- New onset in patient who is <25 or >55 year old
- Sudden onset of hypertension - No Family History - Hypertension with certain electrolytes abnormalities - Failed Empiric therapy Life Style modification Drug Therapy (usually more than 2-3) |
|
|
Causes of secondary hypertension:
|
Renovascular disease
Renal parenchymal disease Polycystic kidneys Aortic coarctation Sleep apnea Pheochromocytoma Primary aldosteronism Cushing syndrome Parathyroid disease Hypo/hyperthyroidism Exogenous causes (Drugs) |

