![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
52 Cards in this Set
- Front
- Back
|
What are enzymes? What is their function? |
Enzymes are proteins and with some exceptions as RNAs that catalyse (increases the rate of) biological reactions at high enough rates to sustain life |
|
|
What protein level of structure do enzymes usually function in |
Tertiary and quaternary structure |
|
|
Two main characteristic of enzymes: As catalysts,enzymes are u__________ by the reaction they catalyse• as catalysts, enzymes increase the ________ of (kinetics) of a reaction but don’t change reaction thermodynamics (favourability) or __________. |
unchanged rate directionality |
|
|
Enzymes greatly accelerate the rate of reactions – with some phenomenal increases. What is an example of a protein that enhances a reaction? How much more is the half life enhanced by? |
e.g. Digestion in the gut Half life expected 2500 years without catalyst. The digestive enzyme carboxypeptidase (PEP) enhances the reaction (t1/2) by 1013 fold to 0.005 s |
|
|
What do transferases do? |
Transferases; catalyse transfer of a functional group from one molecule to another |
|
|
What do hydrolases do? |
Hydrolases; catalyse covalent bond cleavage via the addition of H2O |
|
|
What do Isomerases do? |
Isomerases; catalyse isomerisation (rearrangement) reactions |
|
|
What do lyases do? |
Lyases; catalyse bond breakage removing a chemical group |
|
|
What do ligases do? |
Ligases; catalyse reactions joining two molecules |
|
|
What are isozymes? |
Isozymes are closely-related (non-identical)enzymes that catalyse the same reaction butin different tissues or cellular locations. Theyhave similar amino acid sequences (encodedby different genes) |
|
|
What is an example isozyme? |
e.g. – Lactate dehydrogenase; heart (H) and muscle (M) Hexokinase (I-III, most tissues) and glucokinase (hexokinase IV) liver |
|
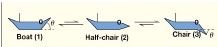
Free energy of a reaction: Out of the reactant boat(1) and the product chair(3) which is the more favourable direction? Why? |
The product chair (3) has a lower free energy than the reactant boat (1), so the overall process/reaction is favourable in the right direction |
|
|
Free energy of a reaction: The reaction proceeds via a free energy barrier (activation energy) to a half-chair transition state TS* (2) Is this unstable or stable? Why? |
The reaction proceeds via a free energy barrier (activation energy) to a half-chair transition state TS* (2) which is unstable (strained) and shortlived |
|
|
What class of reaction is this? |
Isomerases |
|
|
What is a peptide bond |
a stable covalent bond |
|
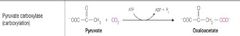
What class of reaction is this? |
Ligases |
|
|
Enzymes (usually) act specifically on one, or a similar group, of ...... |
Substrates (reactants) |
|
|
What is an example of an enzyme acting specifically on one or more substrate (reactants?) |
the enzyme a-glucosidase hydrolyses maltose to 2 glucose molecules, but not cellobiose, owing to the different glycosidic bond (a linkage vs b) even though both maltose & cellobiose are disaccharides of glucose.
|
|
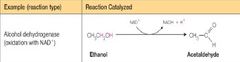
What class of enzyme reaction is this |
Oxidoreductase |
|
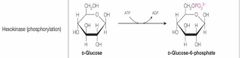
What class of enzyme reaction is this? |
Transferases |
|

What class of enzyme reaction is this? |
Hydrolases |
|
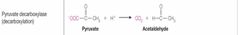
What class of enzyme reaction is this? |
Lyases |
|
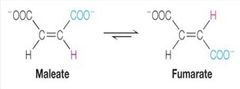
What kind of reaction is this? |
isomerisation (enzyme isomerases) |
|
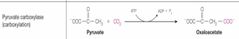
What class of enzyme reaction is this? |
Ligases |
|
|
What are Isozymes (Isoenzymes)? |
Isozymes are closely-related (non-identical) enzymes that catalyse the same reaction but in different tissues or cellular locations. They have similar amino acid sequences (encoded by different genes) |
|
|
Define isomers |
Isomers are molecules which contain the same chemical formula but have different bonding/shape or orientation. |
|
|
what are the three main types of isomers? |
structural isomers cis trans isomers (stereoisomers) enantiomers (stereoisomers) |
|
|
Define stereoisomer |
Each of two or more compounds differing only in the spatial arrangement of their atoms. |
|
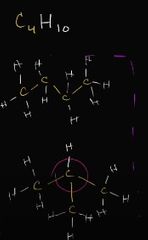
what class of isomer is this |
structural isomer (different bonding) |
|
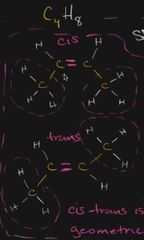
what class of isomer is this |
cis trans (different shapes) |
|
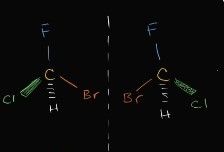
what class of isomer is this |
enantiomers (different orientation) |
|
|
What is an example of an isoenzyme |
Lactate dehydrogenase; heart (H) and muscle (M)can see in your blood if much has leaked out todetermine if you had a heart attack |
|
|
Free energy reaction: what is the highest energy state conformation out of boat 1/half chair 2/chair 3? |
Half chair - The reaction proceedsvia a free energy barrier (activation energy) to a half-chair transition state |
|
|
Factors that can affect enzyme activity include |
pH, temperature, concentration, activators and inhibitors |
|
|
Effect of a catalyst on activation energy: Enzymes stabilise the transition state how? How is this causing reactant molecules to reach a lowered transition state energy? |
Enzymes lower the activation energy, achieving an increased rate as more reactant molecules now have sufficient energy to reach the lowered transition state energy |
|
|
True or false? Not all the glucose molecules at any one time willhavesufficientenergy for the reaction to occur (to go over the hilland form the chair formation) |
True |
|
|
Unjumble this! What is the correct order of enzyme catalysis? -> E+P -> ES -> E+S -> EP |
E+S -> ES -> EP -> E+P E+S=enzyme substrate ES =enzyme substrate complex EP= enzyme products complex E+P = enzyme products |
|
|
How do enzymes provide an ideal chemical environment for a catalysed reaction? |
Providing a chemical environment (e.g. hydrophobic) that is different to aqueous solutionthat allows the reaction to proceed more readily |
|
|
How do enzymes lead to more frequent productive collisions for a catalysed reaction? |
Positioning substrate(s) S in the correct orientation (geometry and orbital overlap) leading to more frequent productive collisions for the reaction |
|
|
Catalysts promote the reaction by enzyme amino acid and/or c______ groups of the correct chemistry |
cofactor |
|
|
Enzyme stabilises the transition TS more than the enzyme-substrate complex (ES). If ES is stabilised too greatly the activation energy is increased/decreased? |
increased |
|
|
The formation of ES tends to be thermodynamically favourable due to what interactions between E and S? |
complementary non-covalent interactions between E and S. |
|
|
What molecular features of enzymes promote transition state stabilisation? |
Enzyme structure |
|
|
What kind of structure is the active site of a an enzyme typically known as? Is this within the enzymes tertiary or quaternary structure? |
Typically a pocket or a groove, in their tertiary structure. |
|
|
What does the active site of an enzyme promote? |
Formation of the transition state and ultimately conversion to product(s) |
|
|
substrate binding to an enzyme: What is the lock and key model? |
where the substrate has an exactstructural and chemical fit to the enzyme active site, like a key in a lock. |
|
|
Substrate binding to an enzyme: What is the induced fit model? |
where the substrate is distorted to thetransition state, with the enzyme also undergoing conformational change(s) upon binding |
|
|
Enzymes bind substrates by what non-covalent interactions? |
hydrogen bonds, salt bridges, Van der Waals & hydrophobic interactions. |
|
|
Why is the induced fit model more consistent with both structural and transition state stabilisation evidence? |
The induced fit model results in transition statestabilisation, promoting a decrease in the activation energy of the reaction |
|
|
An enzyme active site is complementary in what ways to the substrate to help stabilise the reaction transition site? |
An enzyme active site is complementary in shape (structure) and chemistry e.g polarity, charge, hydrophobicity to the substrate. |
|
|
What are the 4 Active site factors affecting transition state (TS) stabilisation: hint: FABD |
- Favourable enzyme–transition state interactions - Altering the reaction pathway to include intermediate states that promote the reaction - Binding of substrate(s) to optimise proximity and orientation for reaction - Distortion (strain) of substrate and enzyme (induced fit) promoting a decrease in activation energy. |
|
|
Enzymes; collision and orientation effects. If NH3 reacting with another reactant molecule - where must NH3 collide within the other reactant molecule to ensure orbital overlap for bond formation? |
Must collide with the reactant molecule at the double bond and in the correct orientation. |

