![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
32 Cards in this Set
- Front
- Back
|
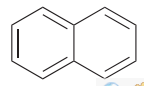
Naphthalene <ˈnæfθəliːn>
|
|
|
|
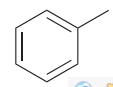
Toluene /ˈtɒluːiːn/
|
|
|
|
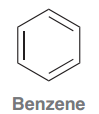
Benzene <benˈziːn>
|
|
|
|
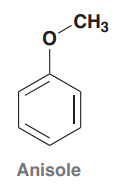
Anisole
|
|
|
|
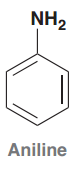
Aniline
|
|
|
|
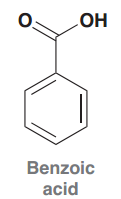
Benzoic acid
|
|
|
|
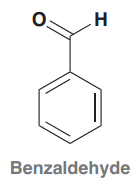
Benzaldehyde
|
|
|
|
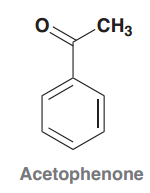
Acetophenone
|
|
|
|
What is Xylene?
|
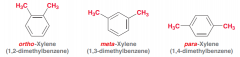
Benzene ring with two methyl substitution
|
|
|
What is TNT?
|
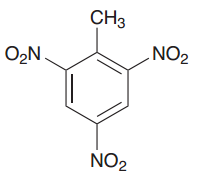
2,4,6-Trinitrotoluene
|
|
|
Is cyclooctatetraene aromatic or anti-aromatic?
|
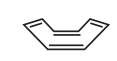
It is non-aromatic (it bends to avoid anti-aromaticity)
|
|
|
Explain Frost circle methods in steps (for drawing the relative position of MO orbitals of a conjugated ring system)?
|

1-Draw a circle / 2-inscribe a polygon, making sure
that one of the connecting points is at the bottom of the circle. / 3- Draw a horizontal line at each point of intersection. / 4-Draw a dotted horizontal line through the center of the circle,and then erase the circle and polygon. /5- Identify all bonding MOs (below the line), nonbonding MOs (on the line), and antibonding MOs (above the line) |
|
|
Draw frost circle for a 4 membered ring?
|
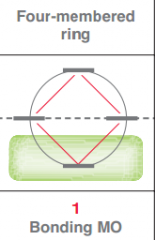
|
|
|
Draw Frost circle for a 5 membered ring?
|
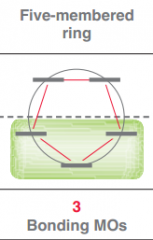
|
|
|
Draw Frost circle for a 6 membered ring?
|
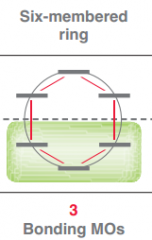
|
|
|
Draw Frost circle for a 7 membered ring?
|
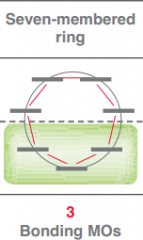
|
|
|
Draw Frost circle for a 8 membered ring?
|
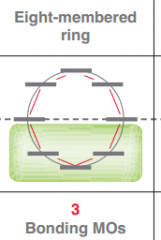
|
|
|
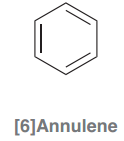
[6]Annulene
|
|
|
|
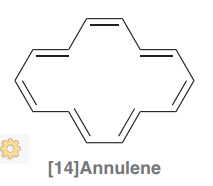
[14]Annulene
|
|
|
|
Is [10]Annulene aromatic?
|
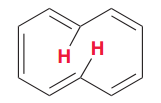
No. It cannot adopt a planar form (hydrogen atoms positioned inside the ring (shown in red) experience
steric hindrance that forces the compound out of planarity) |
|
|
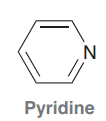
Pyridine
|
|
|
|
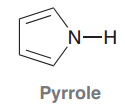
Pyrrole
|
|
|
|
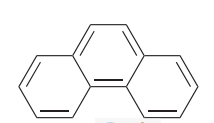
Phenanthrene
|
|
|
|
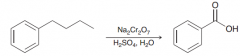
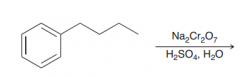
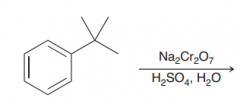
oxidation of alkylbenzenes takes place selectively at the benzylic position. The product of oxidati0n is benzoic acid, irrespective of the identity of the alkyl group.The only condition is that the benzylic position must have at least one proton
|
|
|
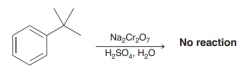
oxidation of alkylbenzenes takes place selectively at the benzylic position. The product of oxidati0n is benzoic acid, irrespective of the identity of the alkyl group.The only condition is that the benzylic position must have at least one proton
|
|
|
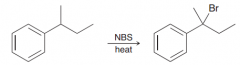
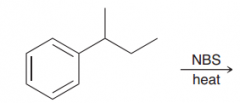
free-radical bromination also occurs readily at benzylic positions. (NBS is good for radical bromination in allilyc and benzylic poisitions)
|
|
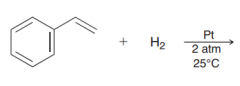
|
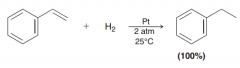
it is possible to selectively hydrogenate a vinyl group in the presence of an aromatic ring
|
|
|
Define Birch reduction?
|
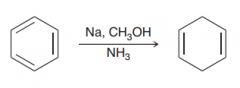
Reduce Benzene moieties by dissolving metal in liquid ammonia. The product is a nonconjugated diene (rather than a conjugated diene) which is fomred via A radical anion
|
|
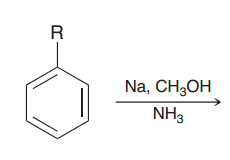
|
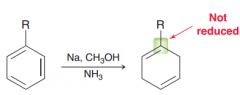
Birch reduction. alkyl benzene is treated with Birch conditions, the carbon atom connected to the
e-donating groups is not reduced ( making the radical anion intermediate unstable) |
|
|
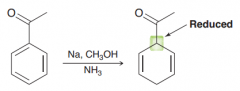
Birch reduction. alkyl benzene is treated with Birch conditions, the carbon atom connected to the
e-withdrawing group is reduced ( making the radical anion intermediate stable) |
|
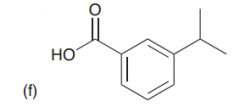
What would be the product of the birch reduction on the following molecule?
|
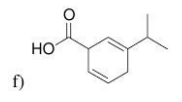
The product is a nonconjugated diene (rather than a conjugated diene) which is fomred via A radical anion. Therefore Carbon attached to a e-donating group is not reduced but carbon to a e-withdrawing group is reduced?
|
|
|
What are the aromatic structure of cyclic compounds from 3,5,6 & 7 membered ring?
|
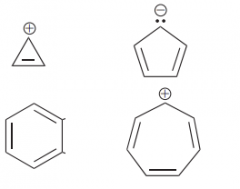
|

