![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
38 Cards in this Set
- Front
- Back
|
1) Under what reaction conditions does the electrophilic chlorination of aromatic compounds usually occur?
A) Cl2, AlCl3 B) Cl2, H2O C) Cl2, CCl4 D) NaCl, H2O E) NaCl, CH3OH |
A) Cl2, AlCl3
|
|
|
2) In electrophilic aromatic substitution reactions a bromine substituent:
A) is a deactivator and a m-director. B) is a deactivator and an o,p-director. C) is an activator and a m-director. D) is an activator and an o,p-director. E) none of the above |
B) is a deactivator and an o,p-director.
|
|
|
3) Which of the following is the best choice of reagents to effect the electrophilic iodination of an aromatic ring?
A) KI, acetone B) I2, CH3CN C) KI, HNO3 D) I2, HNO3 E) none of the above |
D) I2, HNO3
|
|
|
4) Which of the following is an incorrect statement about the bromination of benzene by Br2 and FeBr3?
A) FeBr3 functions to increase the electrophilicity of Br2. B) Formation of the sigma complex is the rate-determining step of the mechanism. C) The carbanionic intermediate is resonance stabilized. D) There are two carbon-containing intermediates in the mechanism. E) The final step of the mechanism is loss of H+. |
C) The carbanionic intermediate is resonance stabilized.
|
|
|
5) Which of the following species is attacked by benzene in the electrophilic nitration reaction?
A) HNO3 B) NO2+ C) NO2 D) NO+ E) N3- |
B) NO2+
|
|
|
6) Which of the following is the strongest activating group in electrophilic aromatic substitution reactions?
A) -CH2CH3 B) -OCH3 C) -CO2CH3 D) -NO2 E) -N(CH3)2 |
E) -N(CH3)2
|
|
|
7) In electrophilic aromatic substitution reactions the hydroxyl group is an o,p-director because:
A) it donates electron density to the ring by induction and destabilizes the meta sigma complex. B) it donates electron density to the ring by resonance and stabilizes the ortho and para sigma complexes. C) it donates electron density to the ring by induction and stabilizes the ortho and para sigma complexes. D) it donates electron density to the ring by resonance and destabilizes the meta sigma complex. E) it withdraws electron density from the ring by induction and destabilizes the meta sigma complex. |
B) it donates electron density to the ring by resonance and stabilizes the ortho and para sigma complexes.
|
|
|
8) The nitration of anisole:
A) proceeds more rapidly than the nitration of benzene and yields predominantly the meta product. B) proceeds more rapidly than the nitration of benzene and yields predominantly the ortho, para products. C) proceeds more slowly than the nitration of benzene and yields predominantly the meta product. D) proceeds more slowly than the nitration of benzene and yields predominantly the ortho, para products. E) proceeds at the same rate as the nitration of benzene and yields predominantly the meta product. |
B) proceeds more rapidly than the nitration of benzene and yields predominantly the ortho, para products.
|
|
|
9) Which of the following compounds will undergo bromination most rapidly using Br2, FeBr3?
A) p-methylacetanilide B) bromobenzene C) acetanilide D) benzenesulfonic acid E) dibromobenzene |
A) p-methylacetanilide
|
|
|
10) In electrophilic aromatic substitution reactions, a -NHCOCH3 substituent on the aromatic ring is:
A) a deactivator and a m-director. B) a deactivator and an o,p-director. C) an activator and a m-director. D) an activator and an o,p-director. E) none of the above. |
D) an activator and an o,p-director.
|
|
|
11) In electrophilic aromatic substitution reactions, a phenyl substituent on the aromatic ring is:
A) a deactivator and a m-director. B) a deactivator and an o,p-director. C) an activator and a m-director. D) an activator and an o,p-director. E) none of the above. |
D) an activator and an o,p-director.
|
|
|
12) Which of the following compounds will undergo Friedel-Crafts alkylation with
(CH3)3CCl, AlCl3 most rapidly? A) toluene B) iodobenzene C) acetophenone D) benzenesulfonic acid E) cyanobenzene |
A) toluene
|
|
|
13) Which of the following compounds will react most rapidly when treated with
CH3CH2Cl and AlCl3? A) benzene B) chlorobenzene C) nitrobenzene D) anisole E) toluene |
D) anisole
|
|
|
17) In electrophilic aromatic substitution reactions the nitro group is:
A) a m-director since it destabilizes the meta sigma complex more than the ortho, para. B) a m-director since it destabilizes the meta sigma complex less than the ortho, para. C) an o,p-director since it stabilizes the ortho, para sigma complex more than the meta. D) an o,p-director since it stabilizes the ortho, para sigma complex less than the meta. E) none of the above. |
B) a m-director since it destabilizes the meta sigma complex less than the ortho, para.
|
|
|
18) In the addition of an electrophile to acetophenone, which of the following best describes the expected mode of reaction?
A) The o,p-positions are most activated to attack by the electrophile. B) The m-positions are most activated to attack by the electrophile. C) The o,p-positions are most deactivated to attack by the electrophile. D) The m-positions are most deactivated to attack by the electrophile. E) All positions (o, m, and p) are equally activated to attack by the electrophile. |
C) The o,p-positions are most deactivated to attack by the electrophile.
|
|
|
19) In electrophilic aromatic substitution reactions, a -CO2H substituent on the aromatic ring is:
A) a deactivator and a m-director. B) a deactivator and an o,p-director. C) an activator and a m-director. D) an activator and an o,p-director. E) none of the above. |
A) a deactivator and a m-director.
|
|
|
20) In electrophilic aromatic substitution reactions, a cyano substituent on the aromatic ring is:
A) a deactivator and a m-director. B) a deactivator and an o,p-director. C) an activator and a m-director. D) an activator and an o,p-director. E) none of the above. |
A) a deactivator and a m-director.
|
|
|
21) Which of the following compounds will not undergo Friedel-Crafts acylation when treated with CH3CH2COCl, AlCl3?
A) toluene B) p-xylene C) anisole D) ethoxybenzene E) benzophenone |
E) benzophenone
|
|
|
22) Which of the following compounds will undergo bromination least rapidly when treated with Br2 and FeBr3?
A) p-methylacetanilide B) bromobenzene C) acetanilide D) benzenesulfonic acid E) benzene |
D) benzenesulfonic acid
|
|
|
23) Which of the following compounds will react least rapidly when treated with CH3CH2Cl and AlCl3?
A) o-xylene B) acetanilide C) toluene D) benzene E) bromobenzene |
E) bromobenzene
|
|
|
25) Which of the following compounds undergoes reaction with HNO3/H2SO4 at the fastest rate?
A) ethylbenzene B) benzenesulfonic acid C) nitrobenzene D) chlorobenzene E) acetophenone |
A) ethylbenzene
|
|
|
30) Which of the following compounds would most likely be used in the preparation of isobutylbenzene from benzene?
A) (CH3)2CHCOCl B) (CH3)2CHCH2Cl C) (CH3)2CHCH2Br D) CH3CH2CH2CH2Cl E) CH3CH2CH2COCl |
A) (CH3)2CHCOCl
|
|
|
31) What intermediate is thought to occur in the elimination-addition nucleophilic aromatic substitution mechanism?
A) radical anion B) radical cation C) quinone D) benzyne E) positively charged sigma complex |
D) benzyne
|
|
|
32) When o-fluorotoluene is treated with sodium amide, the product is:
A) only o-toluidine. B) only m-toluidine. C) only p-toluidine. D) a mixture of o- and p-toluidine. E) a mixture of o- and m-toluidine. |
E) a mixture of o- and m-toluidine.
|
|
|
33) Which of the following compounds is least reactive in the nucleophilic aromatic substitution reaction with NaOH?
A) 2,4-dinitrochlorobenzene B) m-nitrochlorobenzene C) o-nitrochlorobenzene D) p-nitrochlorobenzene E) 3,5-dinitrochlorobenzene |
B) m-nitrochlorobenzene
|
|
|
34) Which toluidine isomers are possible products when m-bromotoluene is treated with NaNH2?
A) ortho, meta, and para B) meta only C) para only D) ortho only E) meta and para only |
A) ortho, meta, and para
|
|
|
35) When 2,4-dinitrochlorobenzene is treated with sodium hydroxide at 100°C followed by protonation:
A) 2,4-dinitrophenol is formed via an addition-elimination nucleophilic aromatic substitution mechanism. B) 2,4-dinitrophenol is formed via an elimination-additon nucleophilic aromatic substitution mechanism. C) 3,5-dinitrophenol is formed via an elimination-addition nucleophilic aromatic substitution mechanism. D) 3,5-dinitrophenol is formed via an electrophilic aromatic substitution mechanism. E) 2,4-dinitrophenol is formed via an electrophilic aromatic substitution mechanism. |
A) 2,4-dinitrophenol is formed via an addition-elimination nucleophilic aromatic substitution mechanism.
|
|
|
38) p-Methoxybenzaldehyde can be prepared from anisole using the Gatterman-Koch formylation. What mixture of reagents is necessary for this process?
A) CO, HCl, AlCl3, CuCl B) CO, SO3, H2SO4 C) CO2, HCl, AlCl3 D) CO2, SO3, H2SO4 E) CO2, HNO3, H2SO4 |
A) CO, HCl, AlCl3, CuCl
|
|
|
26) Rank the following groups in order of increasing activating power in electrophilic aromatic substitution reactions: -OCH3, -OCOCH2CH3, -CH2CH3, -Br.
A) -CH2CH3 < -Br < -OCOCH2CH3 < -OCH3 B) -CH2CH3 < -OCOCH2CH3 < -Br < -OCH3 C) -Br < -OCOCH2CH3 < -CH2CH3 < -OCH3 D) -Br < -CH2CH3 < -OCOCH2CH3 < -OCH3 |
D) -Br < -CH2CH3 < -OCOCH2CH3 < -OCH3
|
|
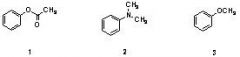
15) Which sequence correctly ranks the following aromatic rings in order of increasing rate of reactivity with chlorine and aluminum chloride
A) 1 < 2 < 3 B) 2 < 3 < 1 C) 3 < 2 < 1 D) 2 < 1 < 3 E) 1 < 3 < 2 |
E) 1 < 3 < 2
|
|
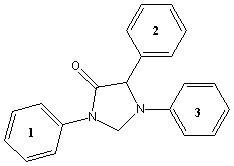
16) Which sequence ranks the aromatic rings in order of increasing reactivity in an electrophilic aromatic substitution reaction (slowest to fastest reacting)?
A) 1 < 2 < 3 B) 2 < 3 < 1 C) 3 < 2 < 1 D) 3 < 1 < 2 E) 2 < 1 < 3 |
E) 2 < 1 < 3
|
|
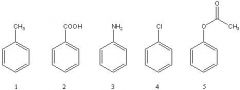
24) Rank the following compounds in order of increasing reactivity towards chlorination with Cl2/AlCl3 (slowest reacting to fastest).
A) 3 < 4 < 2 < 1 < 5 B) 2 < 4 < 1 < 3 < 5 C) 4 < 2 < 1 < 3 < 5 D) 2 < 4 < 5 < 1 < 3 E) 2 < 4 < 1 < 5 < 3 |
E) 2 < 4 < 1 < 5 < 3
|
|
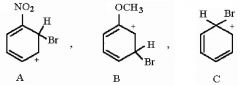
27) Rank the following sigma complexes in order of increasing stability.
A) A < B < C B) B < C < A C) B < A < C D) C < A < B |
A) A < B < C
|
|
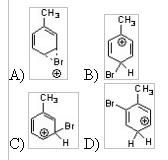
14) Which of the following is an intermediate in the bromination of toluene?
|
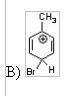
|
|
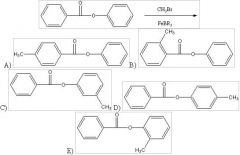
28) Predict the two major products from the following reaction. (Mark all correct responses.)
|
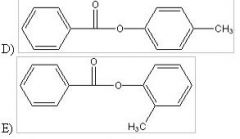
|
|
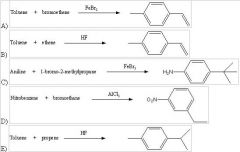
29) Which of the following reactions will actually yield the indicated product?
|

|
|
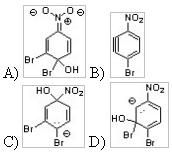
36) Which of the following is an intermediate when 1,2-dibromo-4-nitrobenzene is heated with NaOH in a nucleophilic aromatic substitution reaction?
|
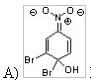
|
|
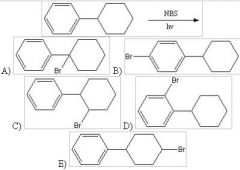
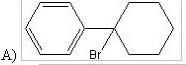
37) Predict the major product form the following reaction:
|
|

