![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
35 Cards in this Set
- Front
- Back
|
Where do the two types of protein synthesis in cells take place? |
1. Cytosolic Ribosomes 2. ER Ribosomes |
|
|
To what organelles do proteins synthesized solely in the cytosol go? |
Mitochondria Chloroplasts (plants) Nucleus Peroxisomes
Also, some proteins synthesized in cytosol stay as cytosolic proteins. |
|
|
To what organelles do proteins synthesized in the Endoplasmic Reticulum go? |
Endoplasmic Reticulum Golgi Apparatus Endosomes/Lysosomes Plasma Membrane
Some of these proteins are eventually completely secreted. |
|
|
What is the cytosol? What does it contain? |
The material that surrounds the organelles and is enclosed by the plasma membrane. It contains many metabolic pathways, protein synthesis, and the cytoskeleton. |
|
|
What is the nucleus? |
An organelle surrounded by a double-membrane nuclear envelope. Communicates with cytosol via nuclear pores that perforate the envelope. Contains DNA and RNA. It's outer membrane is continuous with the ER membrane. |
|
|
What is the Endoplasmic Reticulum? |
A system of interconnected tubes and sacs that extends throughout the cell. Responsible for the synthesis of most lipids, synthesis of proteins, and for distribution of materials to many organelles and the plasma membrane. |
|
|
What is the Smooth Endoplasmic Reticulum? |
It lacks ribosomes and provides membrane lipids for many organelles and the plasma membrane. |
|
|
What is the Rough Endoplasmic Reticulum? |
It has ribosomes attached to its cytosolic surface. Smooth ER can become rough ER. The rough ER has proteins translated directly into its lumen or membrane. Much of this protein is then ferried to other organelles via transport vesicles. |
|
|
What is the Golgi Apparatus? |
It works to modify, sort, and package proteins and lipids in transport vesicles either for secretion or delivery to another organelle. It is the common next stop after the ER |
|
|
What are Lysosomes? |
They are the principal site of intracellular digestion. They are membranous sacs filled with hydrolytic enzymes that carry out the controlled intracellular digestion of both extracellular materials and worn out organelles. Its enzymes function optimally at approx. pH 5. |
|
|
What are mitochondria and chloroplasts? |
In animals, mitochondria provide energy to the cell via ATP synthesis by oxidative phosphorylation. Also play a key role in programmed cell death.
In plants, chloroplasts provide energy to the cell via ATP synthesis and carbon fixation by photosynthesis. |
|
|
What are peroxisomes? |
Small organelles present in the cytoplasm that function in metabolism, especially the oxidation (break down) of long-chain fatty acids and removal of toxic molecules. Present in most cell types of eukaryotes. The very high internal enzyme concentration sometimes crystallizes. |
|
|
What are endosomes? |
Membrane-bound vesicles that receive endocytic vesicles. They either recycle materials back to the plasma membrane or send it to lysosomes for digestion. Basically, they sort endocytosed material. |
|
|
What are signal sequences? |
Sequences typically 15-60 aa's long that help direct proteins to the correct organelle/destination. These sequences are often removed from proteins once the reach their destination. |
|
|
Where are the two places eukaryotic mRNA can be translated? |
On the Rough ER or in the cytosol. |
|
|
What is the default location for proteins without signal sequences? |
The cytosol |
|
|
How are proteins imported into the nucleus? |
1. Nuclear localization sequences in "cargo" proteins (lysine/arginine rich, typically on N Terminal tail) bind to importins, which direct them through the nuclear pore. 2. Nuclear import receptors recognize NLS and interact with cytosolic fibrils of pore to direct protein. 3. Ran-GTP protein required to dissociate protein from importin. 4. GTP hydrolysis exports importin back to cytoplasm and releases it from Ran. |
|
|
Is the tertiary or quaternary structure of a protein disrupted during nuclear import or export? |
No. Proteins remain folded during transport, and even assembled ribosomal subunits can pass through without trouble. |
|
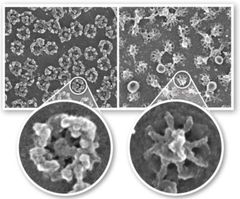
What is this an image of? |
Left is cytosolic side of nuclear pore. Right is nucleus side (inside) of the nuclear pore. (Electron micrograph?) |
|
|
What is the nuclear pore made up of? |
A large complex of nucleoporin proteins. Molecules pass through a gel-like network of nuclear fibrils with a nuclear basket below to get into the nucleus. |
|
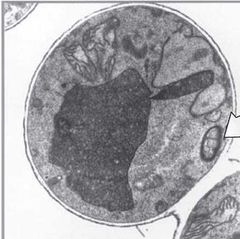
What is this an image of? |
A TEM of a peroxisome with its crystalline core and lipid bilayer. |
|
|
How are proteins imported into peroxisomes? |
Proteins have a perixisomal targeting signal (PTS) of serine-lysine-leucine. They are transported directly into peroxisomes in a folded state. There are two alternative paths.
1. PTS1 path: The Pex5P cytosolic receptor binds to the C terminal tail of the cargo protein for transport through the Docking/Ring complex 2. The PTS2 path: The Pex7P cystolic receptor binds to the N terminal tail of the cargo protein for transport through the same complex.
PTS receptors return to cytosol after transport. |
|
|
How are proteins imported into mitochondria? |
Proteins have an N-terminal mitochondrial signal sequence (MTS) that targets them to the mitochondrial matrix. Proteins are kept unfolded. 1. MTS recognized 1st by receptor on outer mitochondrial membrane (Tom) 2. MTS recognized by 2nd receptor on inner mitochondrial membrane (Tim) |
|
|
How are mitochondrial targeted proteins kept unfolded in the cytosol prior to import? |
They bind to cystolic Hsp70, a chaperonin, which also folds the protein once it has been translocated and had the MTS cleaved off. |
|
|
How extensive is the ER membrane? |
It has 30 times more membrane than the plasma membrane. This is possible due to its many folds. |
|
|
How are proteins translocated into the ER lumen? |
1. Translation of ER proteins begins in the cytosol. These proteins have an ER signal sequence of 8+ hydrophobic aa's. 2. Signal recognition particle recognizes signal sequence and carries ribosome/protein/mRNA complex to ER. 3. SRP receptor embedded in cytosolic side of ER membrane recognizes SRP and binds it. 4. SRP receptor attaches N Terminal ER signal sequence to Translocator Complex and dissociates. 5. Ribosome generates force for translocation of protein through translocator complex as protein is synthesized. 6. Signal peptidase cleaves off signal peptide region of protein to release it into the ER lumen. 7. Protein folds once inside. |
|
|
How are single-pass transmembrane proteins embedded in the ER membrane? |
Same way as lumen proteins with one addition: - A second signal sequence (stop-transfer sequence made up of hydrophobic aa's) halts translocation, leaving the C terminal tail on the outside of the ER and the N Terminal tail on the inside. |
|
|
How does glycosylation occur for ER translocating proteins? |
1. An appropriate Asparagine enters the ER lumen. 2. Intact oligosaccharide chains are held by a lipid called dolichol. 3. Oligosaccharyl transferase catalyzes the transfer of the intact sugar unit to the Asparagine. |
|
|
On which amino acid does N-linked glycosylation happen? |
Asparagine (Asn) |
|
|
On which amino acids does O-linked glycosylation occur? |
Serine or threonine. |
|
|
When does most glycosylation happen? |
As the protein moves through the Golgi Apparatus. |
|
|
How are proteins folded as they enter the ER lumen? |
1. A chaperone protein (BiP) uses hydrolysis of ATP to clamp to a hydrophobic patch of the nascent protein 2. BiP (binding protein) prevents misfolding by slowing folding of the nascent protein. 3. Eventually, ADP replaced by new ATP in BiP, causing it to release and allow the protein to fold. |
|
|
What is a polyribosome? |
Also called polysomes. They occur when many ribosomes attach to the same mRNA to translate it. If the mRNA encodes a protein with an ER signal sequence, the polyribosome will become riveted to the ER membrane due to the polypeptide chains translocating into the lumen. |
|
|
What is a start transfer sequence? |
An internal protein signal sequence that is never removed from a polypeptide. They are used in some transmembrane proteins that pass through the ER membrane multiple times. |
|
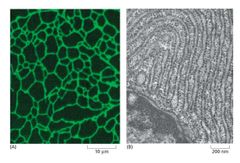
What is this an image of? |
Fluorescent microscopy (left) and TEM (right) of the Endoplasmic Reticulum. |

