![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
61 Cards in this Set
- Front
- Back
|
Fire impacts |
Releases lots of CO2 Clears forest leaving ash layer Post: Soil compaction Impermeable top-layer from ash Loss of slope stability Inc erosion |
|
|
Big bang evidence |
Elemental composition, red shifts, background radiation |
|
|
Atom abundance |

|
|
|
Big bang nucleosynthesis |
-Universe had to cool to 3000k before H and He were stable -Collisions of He produce small amounts of Li, Be and B -No elements heavier than B created till stars appeared |
|
|
Stellar nucleosynthesis |
-gravity leads to matter accumulation and increased local heat -matter + heat = nuclear fusion reactions= C and No formation |
|
|
Explosive nucleosynthesis |
- in large mass stars, tipping point reach where they rapidly collapse -core rebounds sending massive shockwave outward and increasing heat - breakdown of some atoms (Fe) - releases huge quantity of neutrons =formation of heavier elements (>Ni) |
|
|
Iron excess |
- fusion reactions up to iron release energy -fusions reactions to create iron or above consume energy - leads to buildup of iron cores in stars - large star build up> supernova> iron excess |
|
|
Cosmic Ray spallation |
-Free particles (protons) and small atoms (He) from pace smashing into larger atoms-Breaks them into smaller pieces (li, Be, & B) that decay and may be used to date things) |
|
|
Radionuclides |
- large discrepancies between neutrons and protons= usually unstable- always decay with equal or smaller atomic weight |
|
|
Alpha decay |
1 alpha particle = 1 4/2He+ nucleus reduces A (top #) by 4 and Z ( bottom #) by 2 |
|
|
Beta negatron decay |
- occurs when N > P, Z increases by 1, A remains same |
|
|
Position beta decay |
occurs when N < P, Z decreases by 1, A remains same |
|
|
Electron capture beta decay |
occurs when N < P, Z decreases by 1, A remains same |
|
|
Damage potential of radiation types |
Alpha- largest, most dangerous, easily stopped (ex cannot cross skin barrier) Beta- much smaller and less damage than alpha, but travel further/penetrate deeper Gamma- least damage, but penetrates through almost everything
|
|
|
How many moles of iron in 550g of pyrite, FeS2? |
Fe = 55.845 amu, S = 32.06 amuFeS2 = 55.845+2*32.06 = 119.965 amuMass PercentFe = 55.845/119.965 = 0.4655 * 100 = 46.55%S = (2*32.06)/119.965 = 0.5345 * 100 = 53.45%550 * 0.4655 = 256.025 g Fe à 256.025/55.845 = 4.58 moles Fe in 550 grams of pyrite |
|
|
Radiometric dating assumptions |
1.The decay constant (λ) is well-constrained and has not changed over geologic time2.Measurements of D and N are accurate and representative of the sample being dated3.The system has remained closed to the parent and daughter nuclei since it formedD*/N ratio due only to radioactive decay4.D0 can be estimated realistically\ |
|
|
Rb/Sr dating |
Half-life: 4.88*10^10 yearsRb-87 substitutes for K, commonly/easily in igneous rocks |
|
|
Isochron plot |

Isochron Plots: (Sr-87/Sr-86) = (Rb-87/Sr-86)*((e^λt)-1)+ (Sr-87/Sr-86) y = m * x + b |
|
|
U/Pb dating |
Most precise radiometric dates ( because zircons and 2 radioactive U isotopes)-> less than 1% uncertainty= +/- 10s of thousands to millions of years !->In Zircon (ZrSiO4), U substitutes for Z-> Zircon actively rejects Pb, all Pb in zircon is the daughter product*U-238 → Pb-206 half-life: 4.468*10^9 years =99.27% UU-235→ Pb-207 half-life: 7.04*10^8 years =0.72% U |
|
|
Concordia/Discordia |
Concordia: ratio daughter/parent pairs should form specific line Discordia:Line indicating age of formation and major heating resetting event |
|
|
Radiocarbon dating |
Carbon-12 Stable Carbon-13 Stable Carbon-14 Unstable, half-life: 5730 years -> N-14Used to date carbon-bearing sources younger than 60,000 years (max 10 ½ lives) |
|
|
C-14 production |
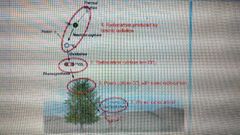
Cosmic ray intensity (prod. Increase with solar activity)Draw of charged particles to the poles = more C-14 at poles (due to earth’s magnetic field) |
|
|
Suess effect |
fossil fuels flooding atmosphere with stable CO2-> relative abundance of C-14 drops |
|
|
Devries effect |
increase in C-14 abundance due to changes in Earth’s magnetic field |
|
|
Nuclear testing |
releases burst of neutrons inducing C-14 production |
|
|
Fractionation |
“Differences in the relative abundance of different isotopes of an element between two reservoirs”Isotope fractionation-Preferential movement of heavy versus light isotopes largely dependent on chemical bonding and mass (often mass-dependent)-Larger proportional mass difference = larger fractionationAt given temp:-Lighter isotopes have more kinetic energy than heavy isotopes -Lighter isotoper easier to escape system -Heavier gets captured in crystalline solid |
|
|
Stable isotope fractionation |
Uses H, C, N, O, & SLarge mass diff between isotopesLess common stable isotope still abundant enough to measure very accurately These elements make up majority of biological & mineral objectsUseful for reconstruction environmental change, magma sources, digenetic processes, biological pathways |
|
|
Fractionation factor |
α(A-B) = R(A) / R(B) A & B are diff reservoirs, R is ratio of heavy to lightVaries with temp, chemical composition, crystal structure/chemical bonds, & pressure |
|
|
Delta notation of fractionation |
Directly compare samples by differences between samples and standards in delta notation δXsample = {(Rsample – Rstandard) / Rstandard } * 1000Units: 0/00 (aka parts per mil) |
|
|
Water isotopologues |
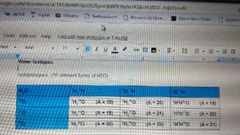
|
|
|
Types of water fractionation |
Equilibrium Fractionation - reaction of two reservoirs with different compositions mixing to reach chemical equilibrium ( ex: oceans behave like equilibrated systems)Kinetic Fractionation - unidirectional exchange across systems Biologically mediated pathways & evaporation |
|
|
Delta notation of ocean water for 2H |
α(ow-wv) = (2H/1H)ow / (2H/1H)wv = 1.074Ocean water enriched 74°/°°in 2H compared to water vapor above ocean |
|
|
Delta notation for ocean water of 18O |
α(ow-wv) = (18O/16O)ow / (18O/16O)wv = 1.0092Ocean water enriched 9°/°°in 18O compared to water vapor above ocean |
|
|
Temperature fractionation relationship |
Fractionation more pronounced in slow reactions ( at poles/ where it’s cold)-> cold regions concentrate light isotopes |
|
|
Rayleigh Distillation |
R = R0f^(α-1)R0 = initial 18O/16O or 2H/1H ratio of water vaporR = 18O/16O or 2H/1H ratio of water vapor after precipitationα = (18O/16O)rain/ (18O/16O)vapor or (2H/1H)rain/ (2H/1H)vaporf = atomic fraction of water vapor remaining in system compared to original amountf = 1 before any precipitation, f = 0 when no water vapor remains |
|
|
Global meteoric water line |
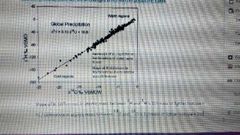
Because precipitation is largely sourced from the same reservoir (oceans) there are linear changes in δO and δH across the Earth (1/1)/(2/16)= 8 |
|
|
Isotope forensics equation |
δX = δX0 + Ɛln(f)^Calculates delta of isotope change of either part of systemδX0 is the initial element’s deltaƐ is the enrichment factor (- values are for calculating delta of remaining low-energy phase, + values are for calculating delta of remaining high-energy phase)f is the fraction of original source remaining |
|
|
Review horse case study |
N/a |
|
|
Marine isotopes |
Paleo temperatures: Changing isotope ratios at a given location should represent changes in temperatureSources of Oxygen often found in calcite-bearing organisms |
|
|
Marine isotopes and u/th dating |
Intermediate decay product on the way to Pb (lead)Th not soluble so all Th in limestone is decay product of U only |
|
|
Marine isotopes 18O record source |
Corals, Conodonts (extinct eel-like vertebrates), forams(1-celled organisms w calcite shell) |
|
|
Marine isotopes biological effects |
Diff species of same group live in diff environments (benthic vs pelagic zones)Within species, individuals in modern day migrate oftenChanges in environment over the course of life Ex: in-shore nursery to offshore life |
|
|
Forams and marine isotopes |
Planktic forams can be found over the entire ocean, not just nearshore making them idealLarge sample size to testLiving/ closely-related species so fractionation can be studied |
|
|
CCD |
CCD is depth of balance between CaCO3 supply and dissolution rates(modern depth ~4,500 m)Below CCD there is more CO2 which acidifies the water |
|
|
Heavy 18O= |
= Ice age - light 18-O is in the ice so seawater becomes heavier |
|
|
Neodymium |
144-Nd is unstable (2.229*10^15 yr), 143-Nd is stableHalf-life ratios driven by fractionation Values reported as Ɛ(Nd)ƐNdsample = {(143Ndsample/144Ndsample – 143NdCHUR/144NdCHUR )/ 143NdCHUR/144NdCHUR } * 10000CHUR = Chondrite Meteorite (Standard ratio) |
|
|
Sea Level Proxy |
ƐNd dependent on sources ( continental vs magmatic)ƐNd used as proxy based on:Nd residence time is shorter than inter-ocean basin mixingƐNd negatively correlated with age because of evolution of Earth’s mantle and radioactivity of SmYounger crust has higher ƐNd valuesOlder crust has lower ƐNd valuesSea level drop → more exposure & weathering of continents negative shifts in ƐNdSea level rise → less exposure & weathering of continents positive shifts in ƐNdIssuesNd measuring craton versus magmatic input with assumption that sea level is the major controlBut craton source dependent on sediment supply rate which is influenced by local basin topography, climate, and relation of sample to delta frontsHigh degree of spatial variation along a coastline |
|
|
Redox state |
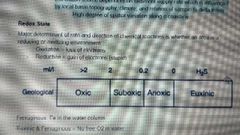
|
|
|
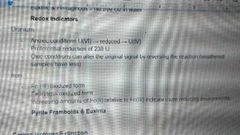
Redox indicators |
|
|
|
Carbon isotopes/extinction |
Carbon is the 4th most common element (50% all living tissue)Largest reservoirs of carbon are sedimentary rocks |
|
|
Carbon flux |
Air → biomass (photosynthesis)Biomass → air (cellular respiration)Biomass → rocks (organic matter burial)Air → Oceans (spontaneous gas exchange) |
|
|
Carbon residence times |
Atmosphere residence time ~5 yearsBiological residence time ~10 yearsEarth residence time >10 million years |
|
|
Carbon cycle feedback loop |

|
|
|
Broken feedback loop |
Burning of fossil fuels releasing CO2 at a rate 100-300x faster than pre-industrial levelsMuch faster than negative feedback loop can keep up Leads to runaway greenhouse effect |
|
|
Mass extinctions and climate change |
All mass extinctions associated with major climate changeEnd-Ordivician (massive cooling)Permian (6-10°C increase) >90% species extinct |
|
|
Isotope excursions |
Carbon: Earth’s carbon system relatively robust to changes in δ13C (± 2 o/oo considered significant over 100,000 yr)Negative isotopic excursions have coincided with two mass extinctions (end-Permian & Late Triassic) |
|
|
Negative isotope excursions |
Negative isotopic excursionsMake reservoir lighterIncrease in carbonate prod and burialMethane hydrate releaseoutgassing from LIPs |
|
|
Positive isotope excursions |
Cooling events/ reservoir heavierDecrease carbonate production and weathering (sea level drop)Burial of organic matterOrganisms are light but as they accumulate in sediment, they leave behind an increasingly heavy ocean reservoir for later organisms Postive isotopic excursions associated with two mass extinctions (end-Oridivician, Late Devonian) |
|
|
Siberian Traps |
Cover an area ⅔ the size of continental United States Erupted in Pulses ~250 myaWould have pumped enormous amounts of CO2 into the atmosphere |
|
|
Baking carbon |
Large field of intrusions in carbon-rich rocks leads to release of CO2 in far greater quantities than from eruptionsAlso occurred during Triassic extinction and possibly part of K-Pg extinction story |
|
|
Magnitude excursion vs extinction |

|

