![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
92 Cards in this Set
- Front
- Back
|
List the main categories of blood cells, with additional nomenclature and approx relative quantities.
|
Red blood cells, erythrocytes, 4-6 x 10 to the 6 / uL (45% blood volume)
Platelets, thrombocytes, 1.5-4 x 10 to the 5 White blood cells, leukocytes, 4-11 x 10 to the 3 (<1% blood volume) |
|
|
Classify white cell types.
|
Granulocytes and Agranulocytes.
Granulocytes include neutrophils (40-70%), eosinophils (1-6%), basophils (<1%) Agranulocytes include monocytes (2-6%) and lymphocytes (20-40%) |
|
|
Give the proportions of different types of white cell.
|
Double amount of neutrophils to lymphocytes (60%-30%)
Roughly the same amount of eosinophils and monocytes (2-5%) Very few basophils (<1%) |
|
|
Classify white cells as either myeloid or lymphoid.
|
Neutrophils, eosinophils, basophils and monocytes are myeloid
Lymphocytes are lymphoid. |
|
|
Describe a red blood cell.
|
Bi concave disc, 7uM in diameter, no nucleus/organelles
|
|
|
How long do red blood cells live for?
|
120 days normally.
|
|
|
What is a reticulocyte?
|
A juvenile red blood cell, has lost it's nucleus but may contain some remaining organelles.
|
|
|
What is the main function of platelets?
|
Haemostasis and clotting (hence thrombocytes)
|
|
|
Describe a platelet.
|
2-3uM in diameter, bi-convex disc, normal organelles and granules containing clotting factor 4, serotonin and some chemotaxins for monocytes and neutrophils.
|
|
|
How long does a platelet live for and how are they formed?
|
8-10 days. Formed from fragmentation of a megakaryocyte, which grows in bone marrow until it is massive 50-70uM, cytoplasm then demarcated by ER vesicles which then fuse, ejecting the platelets.
|
|
|
Which cell type represents over 90% of granulocytes?
|
Neutrophils.
|
|
|
Describe a neutrophil.
|
12-14uM diamater. Multi-lobed nucleus (2-5 lobes).
Contains 3 granule types, one lysosomes, one with inflammatory mediators, and one with adhesion proteins. |
|
|
How do neutrophils get energy?
|
Not many organelles, rely on glycolysis for energy.
|
|
|
How do neutrophils phagocytose?
|
Primary granules containing lysosomes fuse with ingested micro-organism, killing them with burst of super-oxide/hydrogen peroxide.
|
|
|
What is the primary constituent of pus?
|
Neutrophils.
|
|
|
Describe an eosinophil.
|
12-17uM in diameter with a bi-lobed nucleus.
Big and oval in shape. Contain enzymes, lysosomes and histaminase. |
|
|
Which white cell is heavily involved in the phagocytosis of parasites?
|
Eosinophils.
|
|
|
Describe a basophil.
|
12uM in diameter, bi-lobed nucleus, strongly granulated with heparin, histamine.
|
|
|
How are basophils thought to be related to mast cells?
|
Precursor cell. Both have receptors for IgE and are involved in type 1 hypersensitivity.
|
|
|
Describe a monocyte.
|
Large, 20uM in diameter. Horseshoe or kidney shaped nucleus. Enter tissues and become macrophages.
|
|
|
Describe a typical lymphocyte.
|
Small, 6-9uM, big nucleus leaving just a thin rim of cytoplasm. Divided into B and T cells.
|
|
|
What are large lymphocytes?
|
Either activated B-cells on route to becoming plasma cells, or NK cells.
|
|
|
How does the site of haemopoiesis change in the neonate?
|
In utero, begins in the yolk sac, then moves to liver and spleen after 5 weeks. Towards the end of the second trimester, moves to bone marrow.
|
|
|
Where is erythropoietin synthesised and why?
|
In kidney is response to oxygen shortage.
|
|
|
What are the three main types of arteriosclerosis?
|
Atherosclerosis, progessive disease of the intima
Monckeburg's medial sclerosis, a calcification of muscular arteries Arteriolosclerosis, a thickening of the walls of small arteries and arterioles |
|
|
What are the major predisposing factors for atherosclerosis?
|
Diet, hyperlipidaemias, hypertension, smoking and diabetes
|
|
|
What are some minor risk factors for atherosclerosis?
|
Obesity, physical inactivity, male, old, family history, stress, oral contrceptives
|
|
|
Describe the 4 stages in the development of an atheromatous plaque.
|
Stage 1 - Lipid found in macrophages of the intima
Stage 2 - Lipid in both macrophages and smooth muscle cells Stage 3 - Formation of a fibrous plaque Stage 4 - Complex plaque, fibrous with a lipid core. |
|
|
What causes the initial formation of a plaque?
|
Some kind of endothelial injury, but there are roles for macrophages, inflammatory mediators, smooth muscle cells and thrombosis.
|
|
|
What causes endothelial injury?
|
Dysfunction - increased permeability, increased monocyte adhesion and and increased endothelial cell replication. Smoking and high BP increases vascular permeability.
|
|
|
Describe the influence of monocytes in atherosclerosis.
|
Monocytes adhesion increased by molecules VCAM-1 and I~CAM-1. Monocytes and macrophages in the intima express a receptor for LDL. They become foam cells as they fill with LDL.
|
|
|
What is the most common form of hyperlipidaemia and is it primary or secondary?
|
Hyperlipoproteinaemia - can be primary (e.g. genetic) or secondary (e.g. nephrotic syndrome, alcoholism, hypothyroidism, and diabetes mellitus)
|
|
|
What is thrombosis and when is a patient at risk of sudden death?
|
A complication of late-stage atherosclerosis. Fibrin-rich thrombus may contribute to plaque formation and luminal encroachment. If the plaque occludes >70% risk of sudden death is significant.
|
|
|
Why might someone with atherosclerosis limp occasionally?
|
Intermittent claudication. A narrowing of the vascular lumen causing loss of blood flow to the extremities, noticeable when walking after sitting down.
|
|
|
What is haemostasis?
|
Body process involved in the prevention of haemorrhage after vascular injury.
|
|
|
What are the 3 phases of clotting?
|
Initial, amplification and propagation.
|
|
|
What natural inhibitors prevent inappropriate clotting and how do they work?
|
Endothelial cell Nitric oxide and prostacyclins (inhibits platelets)
Tissue Factor Pathway Inhibitor (inactivates factor 10 and 7a) Active Protein C (acts with Protein S to inactivate 5a and 8a) Antithrombin (activated by heparin, inactivates thrombin, 9a, 10a, 11a, 12a) |
|
|
Describe primary haemostasis.
|
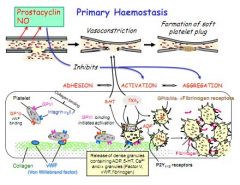
Adhesion, Activation, Aggregation
1. NO and prostacyclins dispersed Adhesion: Platelet receptor GP1b binds collagen vWF Activation: Platelet receptor GP6 activates, releasing ADP, 5-HT, Ca2+ Aggregation: ADP on P2Y and GP2/3 and fibrinogen play a role |
|
|
Describe the initiation phase of clotting.
|
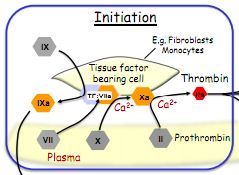
Damaged vasculature exposes tissue factor bearing cells (monocytes/fibroblasts).
Tissue factor combines with factor 7, to give 7a. 7a converts 10 to 10a with Ca2+. 10a converts prothrombin (2) to thrombin (2a) with Ca2+ Thrombin (2a) converts fibrinogen to fibrin which forms the clot. |
|
|
Describe the amplification phase of clotting.
|
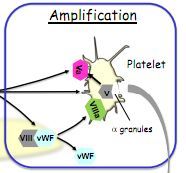
Thrombin splits vWF and factor 8 to factor 8a
Thrombin also splits factor 5 (from platelet alpha-granules) to 5a 5a and 8a attract more platelets and activate them. |
|
|
Describe the propagation phase.
|
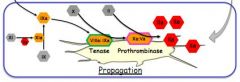
Thrombin also converts 11 to 11a.
11a converts 9 to 9a. 8a (from amplification) and 9a make tenase (10a) Tenase forms 'prothrombinase' (a combination of 10a and 5a) This is a very effective producer of thrombin |
|
|
Describe the process of clot destruction (fibrinolysis).
|
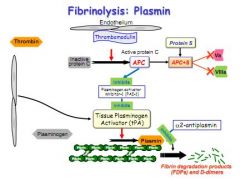
Thrombomodulin activates protein C.
Active protein C and protein S block 5a and 8a Active protein C also inhibits the enzyme (PAI-1) which inhibits Tissue Plasminogen Activator (TPA) Plasminogen is weaved into the structure of any fibrin clot, but is inactive. TPA activates plasminogen but is normally inhibited in clot formation. Active protein C removes this inhibition, allowing TPA to convert plasminogen to plasmin, which breaks down the fibrin clot. |
|
|
How do antiplatelets work?
|
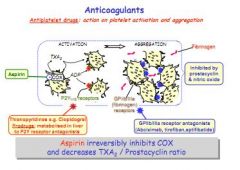
Antiplatelets e.g. Aspirin, inhibit COX. COX is expressed by activated platelets, creating thromboxane, which attracts fibrinogen and other platelets. Other anti-platelets work on platelet or fibrinogen receptors.
|
|
|
How do anticoagulants work?
|
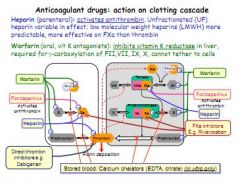
Block an aspect of the clotting cascade.
Heparin activates antithrombin, which inhibits 10a, crucial for the conversion of prothrombin (2) to thrombin (2a). Warfarin is a vitamin K antagonist, vitamin K reductase is vital for production of factors 2, 7, 9 and 10. |
|
|
How would you test for the right dose of warfarin and heparin?
|

|
|
|
Who is predisposed to thrombosis?
|
Virchow's triad:
Vascular damage - causes arterial thrombosis Stenosis (low flow) - causes venous thrombosis Hypercoagulability - thrombocytosis and factor V. |
|
|
What is a 'white thrombus'?
|
Arterial, platelet rich, associated with atherosclerosis/MI. Anti-platelet drugs most effective.
|
|
|
What is a 'red thrombus'?
|
Fibrin rich, associated with veins/DVT. Anti-clotting most effective.
|
|
|
What is haemophilia?
|
Genetic clotting disorder caused by lack of certain clotting factors. Results in prolonged or defective clotting.
|
|
|
Why is vitamin K deficiency a risk for inadequate clotting?
|
Vitamin K an essential precursor to factors 7, 9 and 10 synthesised in the liver. Deficiency causes big problems for clotting.
|
|
|
In addition to haemophilia and vitamin K deficiency, name three other conditions which affect clotting.
|
Antiphospholipid syndrome, von Willebrand factor deficiency and Factor V Leiden.
|
|
|
What is an infarction?
|
An area of ischaemic necrosis produced by occlusion either of arterial supply or venous drainage.
|
|
|
Why is venous infarction less common, and where might you see it?
|
Due to greater number of anastamosing veins. Can get a mesenteric occlusion causing an intestinal infarction. Can also occur in torsion of testes.
|
|
|
How are infarcts classified (colour/location/type)?
|
Red infarcts occur with venous occlusion, in loose tissues like the lung, or tissues with two blood supplies. They are haemorrhagic.
White infarcts occur with arterial occlusion, in solid tissues like the heart, lung and kidney. They are anaemic. Septic infarcts contain bacterial contamination, bland infarcts do not. |
|
|
What are the two types of MI?
|
Transmural (most common) and subendocardial.
|
|
|
Describe a transmural MI.
|
Necrosis of the full thickness of the ventricular wall, caused by occlusion of a single coronary artery.
|
|
|
Describe a subendocardial MI.
|
Necrosis limited to the inner 1/3rd or 1/2 of the ventricular wall, diffuse stenosing, global hypoxia but no plaque or thrombosis.
|
|
|
Describe the structural changes following an MI over time.
|
Initially, ultrastructural changes.
After a few hours - tetrazolium dye detects dehydrongenase 4-12 hours and you see the classic signs of necrosis 12-24hours, beginning of gross changes observable by eye (blue hue) 2-3 days, acute inflammation. 5-10 days, macrophages remove necrotic cardiac muscle cells. 2-4 weeks, granulation, then scarring |
|
|
What complications can be seen post MI?
|
Cardiac rupture - Ventricular free wall, tamponade, interventricular septum (causing shunt), papillary muscles (mitral incompetence)
Pericarditis - Fibrinous/fibrinohaemorrhagic (over necrosis) Mural thrombosis - Due to damaged myocardium (stasis) and endocardium damage Ventricular aneurysm - Late stage; transmural infarct heals into thin fibrous layer which bulges out due to pressure in systole |
|
|
What sorts of things form emboli?
|
Thrombi which break or fragment
Material from ulcerating plaques Septic emboli Tumour fragments Fat globules Air emboli Parenchymal cells (functional parts of organs) |
|
|
Why is DVT a post-operative risk?
|
Form most commonly in legs, due to stasis. Iliac or femoral veins give rise to emboli which can be up to 30cms in length. Block right atrium, pulmonary artery and right ventricle.
|
|
|
What is an atheroma?
|
Build up of cholesterol rich plaques causing stenosis of the artery.
|
|
|
What symptom might you expect from an atheromatous plaque?
|
Angina, or intermittent claudication.
|
|
|
What is angina pectoris?
|
Temporary pain in chest due to imbalance in demand and supply of oxygen.
|
|
|
What are the three types of angina?
|
1. Angina of effort (stable angina)
2. Vasospastic angina 3. Unstable angina (similar to an MI) |
|
|
What causes angina of effort?
|
Stenosis of heart vessel which induces ischaemia when demand rises, e.g. in exercise.
|
|
|
What are the 5 main types of pharmacological treatment for angina of effort and where do they work?
|
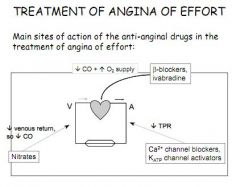
1. B-blockers
2. Ivabradine 3. Nitrates 4. K+ ATP agonist 5. Ca2+ channel blockers |
|
|
What are the two types of beta blockers and how do they help angina of effort?
|
Atenolol - B1 selective
Propanolol - Non-selective Work by reducing sympathetic stimulation of the heart, lowing heart rate and contractility |
|
|
How does ivabradine help treat angina?
|
Reduces I-funny, or If, which is an electrical current used in the SA node to control heart rate.
|
|
|
Describe the cellular mechanism and main sites of action of GTN, route of administration and effect in the treatment of angina.
|
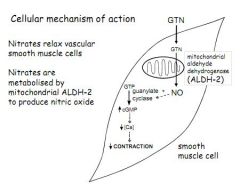
Nitrates relax smooth muscle cells, mostly in veins (lowering CVP, LVEDP and CO) but also in some coronary collaterals (lowering TPR)
GTN is metabolised by Aldehyde Dehydrogenase (ALDH-2) in mitochondria to produce NO NO stimulates Guanylate Cyclase to produce cGMP cGMP lowers [Ca2+] reducing contraction of smooth muscle Administered under the tongue |
|
|
How are nitrites normally administered?
|
Under the tongue or in patches.
|
|
|
Name 2 calcium channel blockers, describe how they work and explain how they help in angina.
|
Verapamil, Nifedipine.
Block Ca2+ entry into smooth and cardiac muscle cells. Main effect in angina is in arteries, reducing TPR Verapamil also reduces heart force |
|
|
Name a K+ATP channel agonist, and describe how it helps in angina?
|
Nicorandil. Opens a K+ channel in smooth muscle cells, hyperpolarises membranes, causes vasodilation and reduction in TPR
|
|
|
How is vasospastic angina treated?
|
With nitrates, Ca2+ channel blockers and K+ channel agonists.
|
|
|
How is unstable angina treated?
|
Like an MI.
|
|
|
What are statins?
|
Drugs which inhibit HMG-CoA reductase, a liver enzyme responsible for production of LDL. Result is liver has to use circulating LDL from plasma.
|
|
|
What two surgical treatments are considered for angina?
|
CABG - Coronary Artery Bypass Graft - essentially using a piece of vein from an arm or leg to replace occluded coronary vessels.
PCI - Per Cutaneous Intervention, essentially a stent. |
|
|
When and why does venous blood thrombose, and what type of treatment is suitable?
|
Usually due to stasis, causes formation of fibrin deposits. Plane flights and atrial fibrillation. Treated with anti-coagulants.
|
|
|
What is heparin and how does it work?
|
Antithrombin activator, which inhibits factor 10a, which is essential for converting 2 (prothrombin) to 2a (thrombin)
|
|
|
What is warfarin and how does it work?
|
Vitamin K antagonist. Stops synthesis of factors 2, 7 and 10 in liver. Takes 1-3 days for full effect.
|
|
|
What is thrombolysis?
|
Administration of fibrinolytic drug which dissolves clots. Used after occlusive strokes and MI.
|
|
|
Give examples of some fibrinolytic drugs.
|
Alteplase, reteplase and streptokinase.
|
|
|
What is the time window for the administration of thrombolysis?
|
3 hours in ischaemic stroke, but up to 12 hours for MI.
|
|
|
Why give an antiplatelet drug?
|
Reduces risk of arterial thrombosis formation.
|
|
|
How does aspirin prevent thrombosis?
|
Aspirin irreversibly inhibits COX, stopping synthesis of TXA and PGI by activated platelets. This is irreversible. Your endothelium repairs and makes new COX, but your platelets cannot.
|
|
|
What is the current treatment for coronary thrombosis?
|
Chewable aspirin and fibrinolytic drug immediately, or coronary angioplasty.
|
|
|
What are the long term goals of post-MI treatment?
|
Lower risk factors - smoking, BP, cholesterol
Exercise - lower BP, less weight to carry around Reduce thrombosis risk - give aspirin in low dose Reduce sympathetic heart stimulation - B-blockers |
|
|
Why would you not give nitrates all the time?
|
Tolerance. Patients build up a resistance to their action, related to inhibition of mitochondrial enzymes. Sporadic administration then is most effective.
|
|
|
Why would you not use a beta-blocker for vasospastic angina?
|
B-blockers are not vasodilators, so would have no effect.
|
|
|
Other than aspirin, list 2 other antiplatelet drugs.
|
P2Y12 receptor antagonists, and GP2b/3a inhibitors
|
|
|
CD4+ and CD8+ T-cells are found in atheromatous plaques, what is their role?
|
Role uncertain
|
|
|
What are the 4 major effects of atherosclerosis on arteries?
|

Narrowing of lumen causing ischaemia
Prolonged ischaemia leading to infarction Possible embolism Weakening of vessel wall, causing aneurysm |

