![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
10 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
|
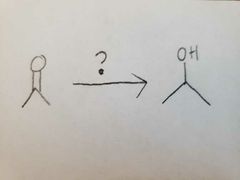
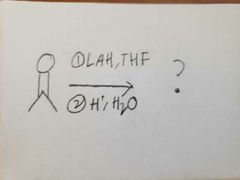
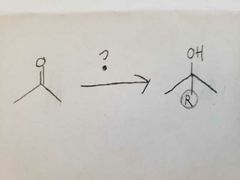
LAH is strong nucleophilic base. It can't work with ethanol so the oxygen has to be protonated separately. |
|
|
|
|
|
|
|
|
|
|
|
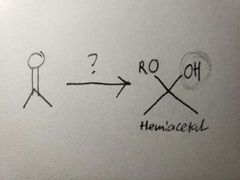
Since the reaction happens in basic conditions, it forms a hemiacetal because the OH group won't leave due to there being a high concentration of OH- already present. |
|
|
|
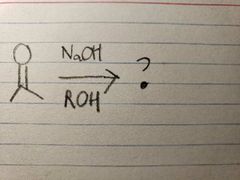
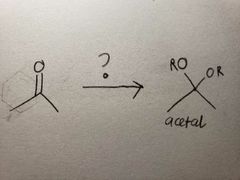
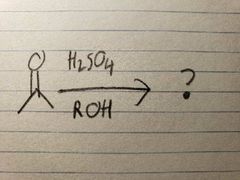
Since it's in acidic conditions, the OH from the hemiacetal form quickly leaves, allowing for the now-more-stable O-R group to take over the final product |
|
|
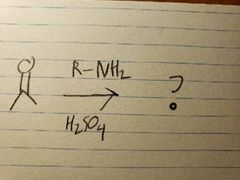
|
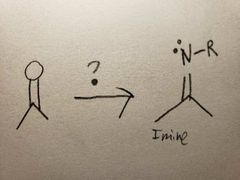
In acidic conditions, the O in the carbonyl turns into an OH group when R-NH2 reacts, so it tends to get replaced kinda quickly. |
|

|
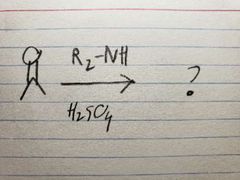
|
The O group turns into an OH group as the amine group attacks, but it's not like the amine can't lose 2 hydrogens, since it only has one, so the molecule settles for the next best thing via resonance: losing an alpha carbon's hydrogen instead, which causes the double bond to form due to extra electrons |
|
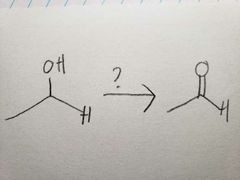
|
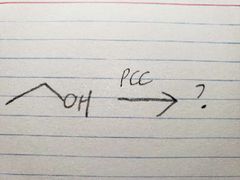
|
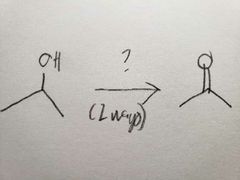
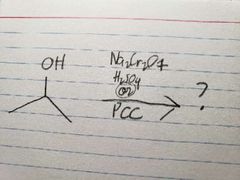
PCC is less powerful than Na2Cr2O7, so it doesn't oxidize all the way |
|
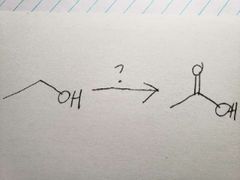
|
|
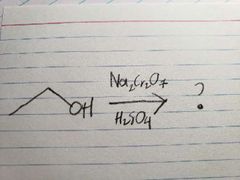
Na2Cr2O7 is such a powerful oxidizer, that it pushed the reaction all the way |
|
|
|
|

