![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
41 Cards in this Set
- Front
- Back
|
How to calculate Degrees of Unsaturation.
|
1 Degree for every 2 H less than Max # H's
-Halides- treat as H's -Oxygen- ignore for calculation -Nitrogen- Remove N and one H |
|
|
Naming Alkenes
|
1. The longest chain gives the parent name.
2. Replace the ane ending with ene. 3. Number the chain so that the double bond has the lowest possible number. 4. Double bonds have priority over every group except OH. 5. The number goes in front of the parent name. 6. The stereochemical prefix goes in front of the entire name. |
|
|
Allyl
|
-CH2CH=CH
|
|
|
Vinyll
|
CH=CH2
|
|
|
Methylene
|
=CH2
|
|
|
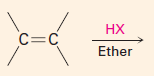
Addition reactions of alkenes
|
Two elements are added to the double bond carbons – one to each carbon.
|
|
|
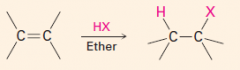
Regiochemistry
|
– which element in the reagent goes to which carbon.
1. Markovnikov regiochemistry – the H+ goes to the carbon of the double bond that has more H’s. Them that has, gits. 2. AntiMarkovnikov regiochemistry – the opposite of Markovnikov 3. The two elements of the reagent must be different for there to be regiochemistry in the reaction |
|
|
Hammond postulate
|
The more stable the intermediate, the faster it forms – there is a lower energy barrier to reach the intermediate state.
|
|
|
Hydride shift
|
If the initially-formed carbocation is 2o and has a 3o or a 4o carbon next to it, it will rearrange to a more stable carbocation. This occurs very quickly. It is a downhill, spontaneous process. If it can happen, IT DOES. Then the nucleophile can bond.
|
|
|
Methyl shift
|
A 4o carbon next to a 2o carbocation results in a methyl shift. A methyl on the 4o carbon leaves it, with its bond, and bonds to the carbocation. This produces a 3o carbocation at the carbon which lost the methyl, and results in a different carbon skeleton.
|
|
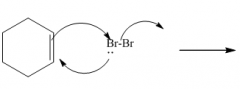
***
|
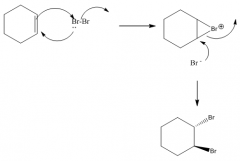
1. Produces a trans-dibromide
2. The mechanism involves a bromonium ion. |
|
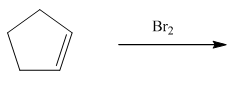
***
|
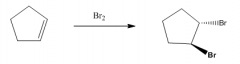
You must show the stereochemistry if 2 chiral centers are formed (a carbon with 2 different groups) otherwise use straight lines:
|
|
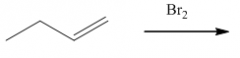
***
|
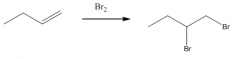
3. You must show the stereochemistry if 2 chiral centers are formed (a carbon with 2 different groups) otherwise use straight lines:
|
|
***
|
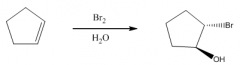
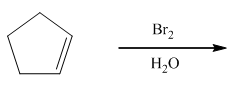
1. Produces a trans-bromohydrin
2. Mechanism involves the bromonium ion, but water is the nucleophile. |
|
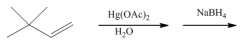
|
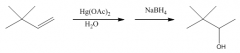
You get Markovnikov addition of H and OH without rearrangement, because there is no carbocation intermediate.
|
|
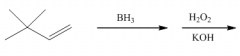
|
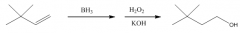
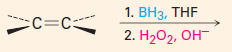
You get anti-Markovnikov addition of H and OH. There is no carbocation intermediate.
|
|
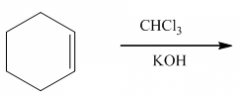
|
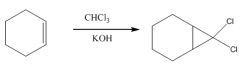
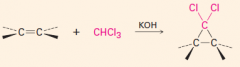
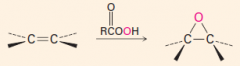
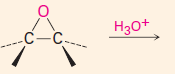
1. This carbene is a very reactive entity that is produced from CHCl3 in KOH.
2. It forms a 3-membered ring where the double bond was. 3. The reaction is stereospecific: |
|
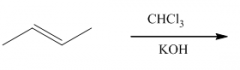
|
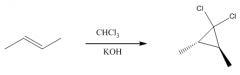
1. This carbene is a very reactive entity that is produced from CHCl3 in KOH.
2. It forms a 3-membered ring where the double bond was. 3. The reaction is stereospecific: |
|
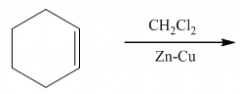
|
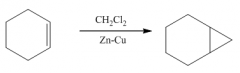
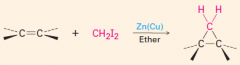
1. This carbine is produced from CH2Cl2 / Zn-Cu
2. The reaction is stereospecific: |
|
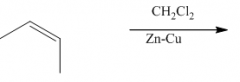
|
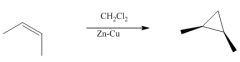
1. This carbine is produced from CH2Cl2 / Zn-Cu
2. The reaction is stereospecific: |
|
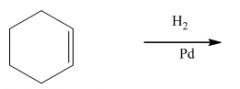
G. Hydrogenation of alkenes
|
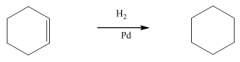
1. The reagent is H2 with catalytic Pd, Ni or Pt.
2. It adds H and H across the double bond, at the same time, so that they go on cis. |
|
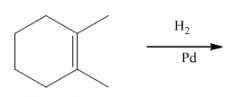
G. Hydrogenation of alkenes
|
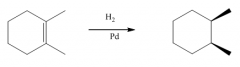
1. The reagent is H2 with catalytic Pd, Ni or Pt.
2. It adds H and H across the double bond, at the same time, so that they go on cis. |
|
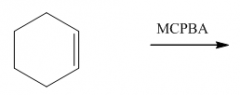
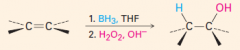
IV. Oxidation of Alkenes
|
|
|
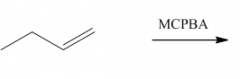
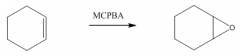
IV. Oxidation of Alkenes
|

|
|
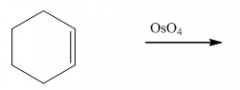
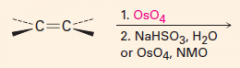
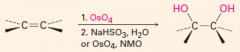
B. To make cis-diols
|
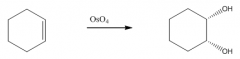
|
|
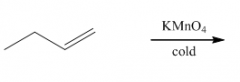
B. To make cis-diols
|
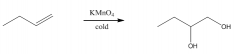
|
|
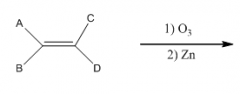
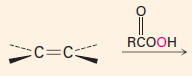
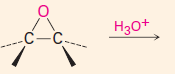
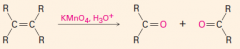
C. To make carbonyl-containing compounds
|
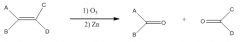
|
|
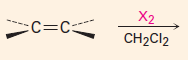
Add X2
*** |
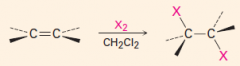
|
|
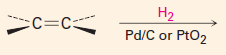
|
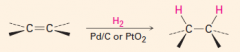
|
|
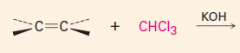
|
|
|
|
|
|
***
|
|
|
|
|
|
|
|
|
|
|
|
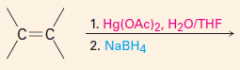
|

|
|
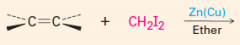
|
|
|
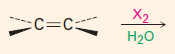
***
|
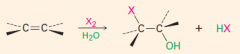
|
|
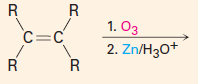
|
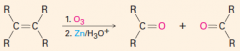
|
|

|
|
|
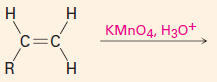
|
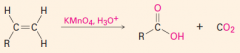
|

