![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
31 Cards in this Set
- Front
- Back
|
Aneurysms
1. Types 2. Causes |
Localized dilation of blood vessel or <3 (aorta most often).
1. True aneurysm: bound by arterial wall components or the attenuated wall of the heart False (pseudoaneurysm): breach arterial wall, extravascular hematoma,bound by extravascular CT (commonly iatrogenic) 2. a. Common - Atherosclerosis-media destruction--- wall thinning Cystic medial degeneration - elastic tissue fragments, cleft-like spaces filled with amorphous extracellular matrix b. Uncommon Congenital defects berry aneurysms, Mycotic aneurysms – secondary to infections (syphilis), Trauma – arteriovenous (fistula),Vasculitis |
|
|
Aortic Aneurysm
1. Location 2. Pathogenesis 3. Morphology 4. Clinical course/complications |
1. Abdominal - most common, b/t renal aa's and bifurcation. Illiac aa's, thoracic aorta, and arch are less common.
2. Atherosclerosis, genetics, male gender, possibly altered CT quality (increased proteolysis of ECM by mmp's) 3. partially thrombus filled, saccular or fusiform. Variants -inflamm., mycotic (2ndary to infection.) 4. can compress ureter, cause tumors. rupture (prop to size), obstruct vessels, embolism. Most pts also have atherosclerosis. ^ risk with ischemic <3 disease, prior stroke. present as ab. mass. Surgical mortality - unruptured 5%, ruptured 50%. |
|
|
Syphilitic (leutic) aneurysm
1. Morphology 2. Clinically |
tertiary syphilis - obliterates small vessels causing aortic complications in vasovasorum.and CNS (meningials).
1. obliterative endarteritis of vasa v. w/ lymphos, plasma cells. Ischemia -- scar -- lost elastic --aneurysm. "Tree barking" contracted. scars. Aortic valve ring dilation -- insufficiency -- hypervol. -- hypertrophy LV 2. Thoracic most common location. Sx due to - push on mediatinum, diff. to breathe and swallow, cough, pain from eroding bone, <3 Disease, rupture. Most die of HF from aortic valve failure. |
|
|
Aortic Dissection
1. dissection def. 2. Pathogenesis, who? 3. Morphology 4. Course |
1. blood in wall of vessel, separation of vessel walls (media), can rupture.
2. HTN Men age 40-60 or younger pts with CT abnormalities (marfans). (also preggers, trama, iatro) Intimal tear starts it. 3. Intimal tear (near aortic valve) rips down wall anterograde. false lumen in middle/outer thirds. Ruptures "out" or renters arterial lumen (double barrell) Atherosclerotic plaque and stop dissection. Marfans most common cause of cystic medial degeneration. 4. depends on level. Type A - comon, serious, ascending aorta, can include abdominal. Type B distal to subclavian. DeBakely Type I ascending + descending. Type II ascending only Type III descending only Sx - sudden excrutiating CP, radiates back and downward. Die rom pericardial, pleural, or peritoneal rupture. Cardiac tamponade, aortic insuff., MI. WIth rapid Dx and treatment 65% survival. Treat with antiHTN and surgery. |
|
|
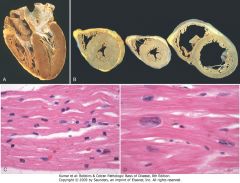
Left ventricular hypertrophy. A, Pressure hypertrophy due to left ventricular outflow obstruction. The left ventricle is on the lower right in this apical four-chamber view of the heart. B, Left ventricular hypertrophy with and without dilation, viewed in transverse heart sections. Compared with a normal heart (center), the pressure-hypertrophied hearts (left and in A) have increased mass and a thick left ventricular wall, while the hypertrophied, dilated heart (right) has increased mass and a normal wall thickness. C, Normal myocardium. D, Hypertrophied myocardium. Note the increases in both cell size and nuclear size in the hypertrophied myocytes.
|
|
|
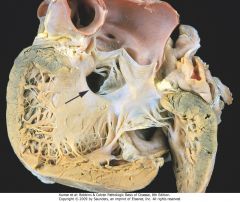
A ventricular septal defect (membranous type), denoted by the arrow
|
|
|
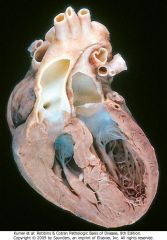
Transposition of the great arteries
|
|
|
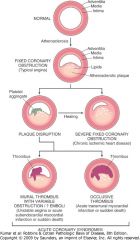
Schematic of sequential progression of coronary artery lesions and their association with various acute coronary syndromes.
|
|
|
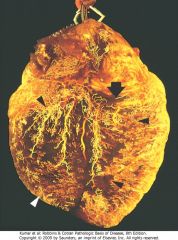
Postmortem angiogram showing the posterior aspect of the heart of a patient who died during the evolution of acute myocardial infarction, demonstrating total occlusion of the distal right coronary artery by an acute thrombus (arrow) and a large zone of myocardial hypoperfusion involving the posterior left and right ventricles, as indicated by arrowheads, and having almost absent filling of capillaries.
|
|
|
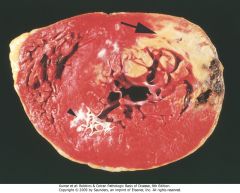
Acute myocardial infarct, predominantly of the posterolateral left ventricle, demonstrated histochemically by a lack of staining by triphenyltetrazolium chloride in areas of necrosis (arrow). The staining defect is due to the enzyme leakage that follows cell death. Note the myocardial hemorrhage at one edge of the infarct that was associated with cardiac rupture, and the anterior scar (arrowhead), indicative of old infarct. Specimen is oriented with the posterior wall at the top.
|
|
|
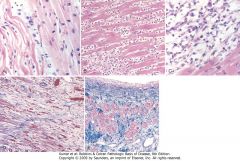
Microscopic features of myocardial infarction and its repair. A, One-day-old infarct showing coagulative necrosis and wavy fibers (elongated and narrow, as compared with adjacent normal fibers at right). Widened spaces between the dead fibers contain edema fluid and scattered neutrophils. B, Dense polymorphonuclear leukocytic infiltrate in area of acute myocardial infarction of 3 to 4 days' duration. C, Nearly complete removal of necrotic myocytes by phagocytosis (approximately 7 to 10 days). D, Granulation tissue characterized by loose collagen and abundant capillaries. E, Well-healed myocardial infarct with replacement of the necrotic fibers by dense collagenous scar. A few residual cardiac muscle cells are present.
|
|
|
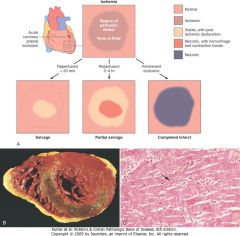
Consequences of myocardial ischemia followed by reperfusion. A, Schematic illustration of the progression of myocardial ischemic injury and its modification by restoration of flow (reperfusion). Hearts suffering brief periods of ischemia of longer than 20 minutes followed by reperfusion do not develop necrosis (reversible injury). Brief ischemia followed by reperfusion results in stunning. If coronary occlusion is extended beyond 20 minutes' duration, a wavefront of necrosis progresses from subendocardium to subepicardium over time. Reperfusion before 3 to 6 hours of ischemia salvages ischemic but viable tissue. This salvaged tissue may also demonstrate stunning. Reperfusion beyond 6 hours does not appreciably reduce myocardial infarct size. B, Gross and C, microscopic appearance of myocardium modified by reperfusion. B, Large, densely hemorrhagic, anterior wall acute myocardial infarction in a patient with left anterior descending artery thrombus treated with streptokinase, a fibrinolytic agent (triphenyl tetrazolium chloride-stained heart slice). Specimen oriented with posterior wall at top. C, Myocardial necrosis with hemorrhage and contraction bands, visible as dark bands spanning some myofibers (arrow). This is the characteristic appearance of markedly ischemic myocardium that has been reperfused.
|
|
|
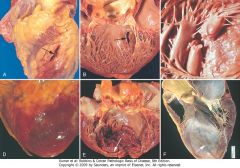
Complications of myocardial infarction. Cardiac rupture syndromes (A-C). A, Anterior myocardial rupture in an acute infarct (arrow). B, Rupture of the ventricular septum (arrow). C, Complete rupture of a necrotic papillary muscle. D, Fibrinous pericarditis, showing a dark, roughened epicardial surface overlying an acute infarct. E, Early expansion of anteroapical infarct with wall thinning (arrow) and mural thrombus. F, Large apical left ventricular aneurysm. The left ventricle is on the right in this apical four-chamber view of the heart.
|
|
|
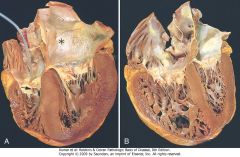
Hypertensive heart disease, systemic and pulmonary. A, Systemic (left-sided) hypertensive heart disease. There is marked concentric thickening of the left ventricular wall causing reduction in lumen size. The left ventricle and left atrium (asterisk) is on the right in this apical four-chamber view of the heart. A pacemaker is present in the right ventricle (arrow). B, Pulmonary (right-sided) hypertensive heart disease (cor pulmonale). The right ventricle is markedly dilated and has a thickened free wall and hypertrophied trabeculae (apical four-chamber view of heart, right ventricle on left). The shape of the left ventricle (to the right) has been distorted by the enlarged right ventricle.
|
|

|
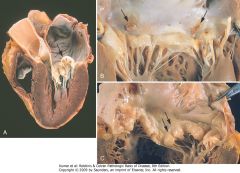
Calcific valvular degeneration. A, Calcific aortic stenosis of a previously normal valve (viewed from aortic aspect). Nodular masses of calcium are heaped up within the sinuses of Valsalva (arrow). Note that the commissures are not fused, as in postrheumatic aortic valve stenosis (see Fig. 12-27E). B, Calcific aortic stenosis of a congenitally bicuspid valve. One cusp has a partial fusion at its center, called a raphe (arrow). C and D, Mitral annular calcification, with calcific nodules at the base (attachment margin) of the anterior mitral leaflet (arrows). C, Left atrial view. D, Cut section of myocardium.
|
|
|
Myxomatous degeneration of the mitral valve. A, Long axis of left ventricle demonstrating hooding with prolapse of the posterior mitral leaflet into the left atrium (arrow). The left ventricle is on right in this apical four-chamber view. B, Opened valve, showing pronounced hooding of the posterior mitral leaflet with thrombotic plaques at sites of leaflet-left atrium contact (arrows). C, Opened valve with pronounced hooding from patient who died suddenly (double arrows). Note also mitral annular calcification (arrowhead). Normal heart valve (D) and myxomatous mitral valve (E) (Movat pentachrome stain, in which collagen is yellow, elastin is black, and proteoglycans are blue). In myxomatous valves, collagen in the fibrosa is loose and disorganized, proteolgycans (asterisk) are deposited in the spongiosa, and elastin in the atrialis is disorganized.
|
|

|
Myxomatous degeneration of the mitral valve. A, Long axis of left ventricle demonstrating hooding with prolapse of the posterior mitral leaflet into the left atrium (arrow). The left ventricle is on right in this apical four-chamber view. B, Opened valve, showing pronounced hooding of the posterior mitral leaflet with thrombotic plaques at sites of leaflet-left atrium contact (arrows). C, Opened valve with pronounced hooding from patient who died suddenly (double arrows). Note also mitral annular calcification (arrowhead). Normal heart valve (D) and myxomatous mitral valve (E) (Movat pentachrome stain, in which collagen is yellow, elastin is black, and proteoglycans are blue). In myxomatous valves, collagen in the fibrosa is loose and disorganized, proteolgycans (asterisk) are deposited in the spongiosa, and elastin in the atrialis is disorganized.
|
|
|
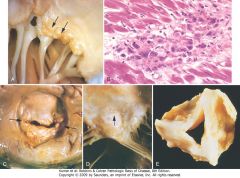
Acute and chronic rheumatic heart disease. A, Acute rheumatic mitral valvulitis superimposed on chronic rheumatic heart disease. Small vegetations (verrucae) are visible along the line of closure of the mitral valve leaflet (arrows). Previous episodes of rheumatic valvulitis have caused fibrous thickening and fusion of the chordae tendineae. B, Microscopic appearance of Aschoff body in a patient with acute rheumatic carditis. The myocardial interstitium has a circumscribed collection of mononuclear inflammatory cells, including some large macrophages with prominent nucleoli and a binuclear macrophage, associated with necrosis. C and D, Mitral stenosis with diffuse fibrous thickening and distortion of the valve leaflets and commissural fusion (arrows, C), and thickening of the chordae tendineae (D). Note neovascularization of anterior mitral leaflet (arrow, D). E, Rheumatic aortic stenosis, demonstrating thickening and distortion of the cusps with commissural fusion.
|
|
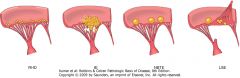
|
Comparison of the four major forms of vegetative endocarditis. The rheumatic fever phase of rheumatic heart disease (RHD) is marked by small, warty vegetations along the lines of closure of the valve leaflets. Infective endocarditis (IE) is characterized by large, irregular masses on the valve cusps that can extend onto the chordae (see Fig. 12-25). Nonbacterial thrombotic endocarditis (NBTE) typically exhibits small, bland vegetations, usually attached at the line of closure. One or many may be present (see Fig. 12-27). Libman-Sacks endocarditis (LSE) has small or medium-sized vegetations on either or both sides of the valve leaflets.
|
|
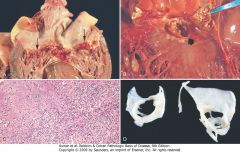
|
Infective (bacterial) endocarditis. A, Endocarditis of mitral valve (subacute, caused by Streptococcus viridans). The large, friable vegetations are denoted by arrows. B, Acute endocarditis of congenitally bicuspid aortic valve (caused by Staphylococcus aureus) with extensive cuspal destruction and ring abscess (arrow). C, Histologic appearance of vegetation of endocarditis with extensive acute inflammatory cells and fibrin. Bacterial organisms were demonstrated by tissue Gram stain. D, Healed endocarditis, demonstrating mitral valvular destruction but no active vegetations.
|
|
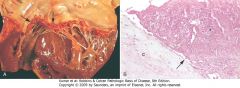
|
Nonbacterial thrombotic endocarditis (NBTE). A, Nearly complete row of thrombotic vegetations along the line of closure of the mitral valve leaflets (arrows). B, Photomicrograph of NBTE, showing bland thrombus, with virtually no inflammation in the valve cusp (c) or the thrombotic deposit (t). The thrombus is only loosely attached to the cusp (arrow).
|
|
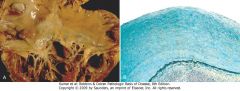
|
Carcinoid heart disease. A, Characteristic endocardial fibrotic lesion involving the right ventricle and tricuspid valve. B, Microscopic appearance of carcinoid heart disease with intimal thickening. Movat stain shows myocardial elastic tissue (black) underlying the acid mucopolysaccharide-rich lesion (blue-green).
|
|
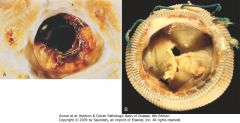
|
Complications of artificial heart valves. A, Thrombosis of a mechanical prosthetic valve. B, Calcification with secondary tearing of a porcine bioprosthetic heart valve, viewed from the inflow aspect.
|
|
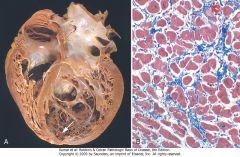
|
Dilated cardiomyopathy. A, Four-chamber dilatation and hypertrophy are evident. There is granular mural thrombus (arrow) at the apex of the left ventricle (on the right in this apical four-chamber view). The coronary arteries were patent. B, Histologic section demonstrating variable myocyte hypertrophy and interstitial fibrosis (collagen is highlighted as blue in this Masson trichrome stain).
|
|
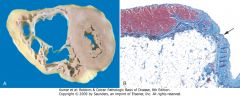
|
Arrhythmogenic right ventricular cardiomyopathy. A, Gross photograph, showing dilation of the right ventricle and near-transmural replacement of the right ventricular free-wall by fat and fibrosis. The left ventricle has a virtually normal configuration. B, Histologic section of the right ventricular free wall, demonstrating replacement of myocardium (red) by fibrosis (blue, arrow) and fat (Masson trichrome stain).
|
|
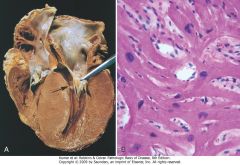
|
Hypertrophic cardiomyopathy with asymmetric septal hypertrophy. A, The septal muscle bulges into the left ventricular outflow tract, and the left atrium is enlarged. The anterior mitral leaflet has been moved away from the septum to reveal a fibrous endocardial plaque (arrow) (see text). B, Histologic appearance demonstrating disarray, extreme hypertrophy, and branching of myocytes as well as the characteristic interstitial fibrosis (collagen is blue in this Masson trichrome stain).
|
|
|
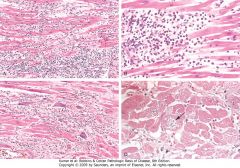
Myocarditis. A, Lymphocytic myocarditis, associated with myocyte injury. B, Hypersensitivity myocarditis, characterized by interstitial inflammatory infiltrate composed largely of eosinophils and mononuclear inflammatory cells, predominantly localized to perivascular and expanded interstitial spaces. C, Giant-cell myocarditis, with mononuclear inflammatory infiltrate containing lymphocytes and macrophages, extensive loss of muscle, and multinucleated giant cells. D, The myocarditis of Chagas disease. A myofiber distended with trypanosomes (arrow) is present along with inflammation and necrosis of individual myofibers.
|
|
|
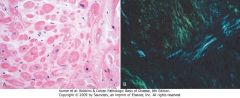
Cardiac amyloidosis. A, Hematoxylin and eosin stain, showing amyloid appearing as amorphous pink material around myocytes. B, Congo red stain viewed under polarized light, in which amyloid shows characteristic apple-green birefringence (compared with collagen, which appears white).
|
|
|
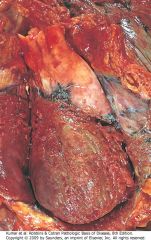
Acute suppurative pericarditis arising from direct extension of a pneumonia. Extensive purulent exudate is evident.
|
|
|
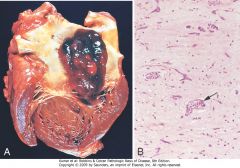
Left atrial myxoma. A, Gross photograph showing large pedunculated lesion arising from the region of the fossa ovalis and extending into the mitral valve orifice. B, Microscopic appearance, with abundant amorphous extracellular matrix in which there are scattered collections of myxoma cells in various groupings, including abnormal vessel-like formations (arrow).
|
|
|
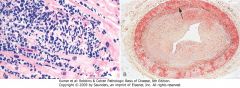
Complications of heart transplantation. A, Cardiac allograft rejection typified by lymphocytic infiltrate, with associated damage to cardiac myocytes. B, Graft coronary arteriosclerosis, demonstrating severe diffuse concentric intimal thickening producing critical stenosis. The internal elastic lamina (arrow) and media are intact (Movat pentachrome stain, elastin black). (B, Reproduced by permission from Salomon RN et al.: Human coronary transplantation-associated arteriosclerosis. Evidence for chronic immune reaction to activated graft endothelial cells.
|

