![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
18 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Periodic table
|
The elements are arranged in order if proton number, vertical column are called groups they have similar properties, horizontal rows are called periods.
|
Arrangement
|
|
|
When was the periodic table created and who has rearranged it
|
In the early 1800 by Dobereiner. Newlands arranged them in order of relative atomic mass. Mendeleev keeping it in the order of relative atomic mass but left gaps for missing elements. Which later scientists discovered.
|
|
|
|
Hazard symbols, table of symbol, meaning of symbol and saftey precaution.
|

|
|
|
|
Group 1 what it is called, and properties
|
Alkali metals.
Low density, low melting/boiling points, shiny when cut but quick to tarnish. Going down the group the reactions get more vigorous. |
|
|
|
What does alkali metals + water make
|
Alkali metals + water - - > hydrogen and alkaline solution
|
|
|
|
Group 7, name and properties
|
Halogens
Going down the group melting/boiling points increase, but become less reactive. Halogen molecules are diatomic - two atoms joined together. |
|
|
|
Atomic particles - table of relative mass and charge
|
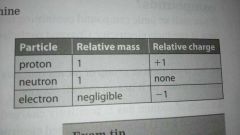
Proton, neutron, electron
|
|
|
|
Atomic structure
|
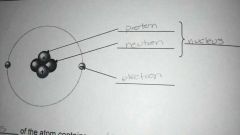
Proton and neutron make up nucleus. Electron orbit the nucleus in a cloud/shell
|
|
|
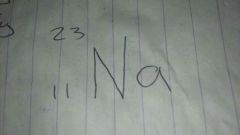
Element mass number and atomic number. Top or bottom?
|
Top number atomic mass
Bottom number atomic number |
|
|
|
How to work out how many electrons/protons an atom contains?
|
Atomic number (bottom number) they are the same number because nucleus is made of equal number of both
|
|
|
|
How to work out how many neutrons an atom contains?
|
Atomic mass (top) - atomic number (bottom) = neutrons
|
|
|
|
Drawing electrons in atoms
|
Use the electrons number and fill the shells 2.8.8.8 formation, filling each shell before moving out to the next shell
|
|
|
|
Elements have a distinctive flame colour, line spectrum
|

When the light from the flame goes through a prism, it makes a line spectrum.
|
|
|
|
What is an ion
|
An electrically charged atom or group of atoms.
|
|
|
|
What are charged ions?
|
They can be either positively or negatively charged. This happens be abuse atoms either lose or gas electrons in order to get a full outer shell.
|
|
|
|
Positively charged ions
|
Lose an electron then positively charged.
|
|
|
|
Negatively charged ion
|
Gains an electron then positively charged.
|
|
|
|
Ion calculations, example is potassium oxide.
|
The total charge on the ions in a formula is zero. Example is potassium is K (+1) and oxygen is I (-2). There has to be two K for one I. So the formula is K2O.
|
|

