![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
165 Cards in this Set
- Front
- Back
|
What do first generation biomaterials share in common? |
- Not originally engineered for biomaterials applications. E.g. sausage casing for kidney dialysis - Just used/designed to be inert (not reactive with the body)
Early biomaterials: iron, brass, gold, glass, wood
<>
Second generation biomaterials: structurally and functionally advanced materials |
|
|
What is the difference between electropositive and electronegative? |
Electropositive: atoms that have one or two more atoms than are required for a full valance shell - Tend to be donors - i.e. become (+) when full
Electronegative: atoms that need one or two electrons for a full valance shell - Tend to be acceptors - i.e. become (-) when full
Both these determine how elements form bonds. |
|
|
What is the difference between organic and inorganic compounds? |
Inorganic: form an inanimate, non-biological origin; do not have carbon-hydrogen bonds
Organic: contain carbon-hydrogen bonds - i.e. a carbon backbone |
|
|
What is the difference between inert and bioactive? *** |
Inert: substance that doesn't cause a reaction - If something is implanted in the body, body will not response via immune response
Bioactive***: implanted material works in combination with existing tissue to trigger the desired response (healing or tissue regeneration) |
|
|
What are the three main classes of biomaterials? |
1. Ceramics 2. Metals 3. Polymers (synthetic and natural)
Also composites - a combination of the above |
|
|
What are ceramics? |
- Inorganic compounds - Contain metallic and non-metallic elements - Inter-atomic bonding is ionic or covalent, which are generally formed at high temperatures - Non-directional bonds - Made from minerals |
|
|
List some advantages and disadvantages** of ceramics. |
Advantages: - Inert or bioactive in body - High wear resistance - High modulus and compressive strengths - Fine aesthetic properties for dental applications
Disadvantages: - Brittle - Low tensile strength - Poor fatigue resistance |
|
|
What must one consider with ceramics? |
Charge and physical size of atoms due to ionic nature.
Usually use radius ratio:
R_cation ------------ R_anion |
|
|
Name some applications of ceramic material. |
- Dental crowns - Knee prostheses - Spinal fusion devices - Inner ear implants (cochlear implants) - Drug delivery devices |
|
|
When are ceramics particularily useful? |
Extremely useful as coatings for metallic implants because coating aids in tissue fixation via porous surface - mechanically interlocks
Considered bioactive ceramics if they establish bonds with bone tissue |
|
|
Compare the two types of point defects. |
Schottky defects: vacancy in both cation and anion, which can be far apart in the material
Frenkel defects: vacancies are created to offside the change in charge caused by an interstitial pair - Due to pair of atoms, not two point vacancies like with Schottky defects
Both are point defects and aim for overall neutrality. |
|
|
What are metals? |
Inorganic materials possessing non-directional metallic bonds with highly mobile electrons
- Closely packed crystal structure (e.g. BBC, FCC, hexagonal, etc.) - Crystalline - and thus have certain shape! - Valuable as load bearing implants (e.g. internal fixation devices or orthopaedic applications, dental implants) - High tensile, fatigue, and yield strengths - Low reactivity and good ductility (e.g. hip implants)
Note: properties depend on processing method and purity of metal |
|
|
Nam three crystal lattice types. |
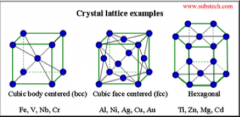
Cubic body centered (BCC) Cubic face centered (FCC) Hexagonal |
|
|
What does the atomic packing factor indicate, and what is the equation?*** |
How much unoccupied space is present in the structure.
APF = (volume of atoms in unit cell)/(total unit cell volume) >1 |
|
|
What is a composite? |
Incorporates the desired characteristics of different materials to meet the stringent demands of living tissue.
E.g. porosity and hardness of ceramics support tissue integration into the tissue/implants interface, but these properties are not important for ligament replacement
Most composite designs combine strength and flexibility by reinforcing a relatively flexible material with a harder, stronger one
In some cases, one or more of these materials may be degradable in order to encourage tissue integration |
|
|
What are polymers? |
Organic materials possessing long chains held together by directional covalent bonds
Consist of small, repeating units strung together in long chains
Can be natural or synthetic
E.g. plastic garbage bags, rubber tires, DNA
In many materials, processing conditions can induce the polymer chains to link with each other along the length of the chain to produce a wide variety of mechanical properties. Parameters are easily varied in order to suit current biomedical applications |
|
|
Name and describe the polymerization reactions. |
Addition: whole molecule incorporated into polymer
Condensation: part of molecule is kicked out when monomer becomes part of a polymer; produces small molecule like H2O or HCl
Chair growth reaction: monomers become part of the polymer one at a time
Step growth reaction: multiple reaction products are possible |
|
|
What are the properties associated with polymers? |
Thermoplastics: materials that soften, melt, and flow when heat is applied; adhesives solidify when cooled - Majority of familiar plastics - Can be reprocessed
Thermosets: - Hard, strong, rigid - Excellent heat resistance - Cannot be reprocessed - Crystalline: epoxy, phenolic, polyester - Amorphous: rubber, silicone, polyurethane
|
|
|
How can you calculated molecule weight? |
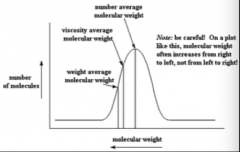
1. Number average molecular weight: Mn = (total weight of all polymer molecules in sample)/(total number of polymer molecules in sample)
Mn - weight / molecules = NxMx/Nx
2. Weight average molecular weight:
Mw = CxMx/Cx = (NxMx^2)/(NxMx)
where N is the number of moles in sample with mass M and N*M is mass of sample
3. Polydisperisty: indicates diversity of mixture
Polydispersity = Mw/Mn >= 1
|
|
|
How can you determine how crystalline a polymer is? |
% crystallinity = pc(ps-pa)/ps(pc-pa)
where ps is the density of sample, pc is density of polymer in crystalline form, and pa is density in amorphous form |
|
|
What does the stress-strain plot indicate? |
Slope is the modulus of elasticity (Young's modulus)
If slope is linear, the process is an elastic deformation |
|
|
What consitutes a surface vs. interface? |
Interface: boundary region between two adjacent bulk phases - Includes L/L, S/S, L/S, L/V (vapor), S/G
Surface: edge or an object or material - Includes S/L, S/G and L/V
SEE IMAGE THAT GOES ALONG WITH THIS |
|
|
What is meant by 'surface free energy'? |
Molecules at the surface are in a state of higher free energy than those in the bulk - Mostly due to lack of nearest neighbour interactions at the sruface |
|
|
Name two ways to estimate the surface energies of solids. |
Surface tension of solids is not experimentally accessible.
Two multiple probe approaches to estimate surface energies of solids:
1. Critical surface tension method (Zisman method) 2. "Molecular approach" (Good, Fowkes method)
|
|
|
Describe the Zisman method. |
Problem: high surface energy liquids will not spread on low surface energy solids as this will not lower their excess surface free energy.
Critical surface tension gamma_c is useful measure of surface tension of solid (maybe) - Only works wells if interaction between probe liquids and surface is dominantly dispersive
?????????? |
|
|
What does the contact angle indicate? |
Degree of wetting - i.e. hydrophobicity - which varies with material
For water probe, 102 for Teflon and PTFE, 72 for Mylar and PET, 5 for glass
For Teflon probe, 102 for water, 71 for methylene iodide, 25 for decane |
|
|
Name 6 techniques for surface characterization. |
1. Contact angle technique 2. Electron Spectroscopy for Chemical Analysis (ESCA/XPS) 3. Secondary Ion Mass Spectroscopy (SIMS) 4. Scanning Probe Microscopy and Atomic Force Microscopy (AFM) 5. Scanning Electron Microscopy (SEM) 6. Surface Plasmon Resonance (SPR) |
|
|
What does the contact angle surface characterization technique provide? |
Liquid wetting of surfaces used to estimate energy of surfaces
Spatial resolution: 1mm
Sensitivity: depends on chemistry
Cost: $ |
|
|
What information does the Electron Spectroscopy for Chemical Analysis provide? |
X-rays induce emission of electrons of characteristic energy - Chemical info about surface of solid materials
Spatial resolution: 10-150µm
Sensitivity: 0.1 atom %
Cost: $$$ |
|
|
What information does SIMS provide? |
Ion bombardment sputters secondary ions form surface - Composition, structure, spatial distribution imaging - Destructive technique by design
Spatial resolution: 100 Å
Sensitivity: very high
Cost: $$$ |
|
|
What information does AFM provide? |
IR radiation adsorbed and excites molecular vibrations - Spatial distribution imaging, topography, thickness
Spatial resolution: 10 µm
Sensitivity: 1 mol %
Cost: $$ |
|
|
What is SEM and what does it provide? |
Instrument that scans a sample surface with finely converged electron beam in a vacuum, detects information (signals) produced at that time from the sample, and presents an enlarged image of the sample surface on the monitor screen
Gives information about sample surface.
Spatial resolution: 40 Å
Sensitivity: high
Cost: $$ |
|
|
What is SPR and what does it provide? |
Gives the resonant, collective oscillation of valance elections in a solid stimulated by incident light - Happens when frequency of light matches natural frequency of surface electrons oscillating against restoring force of positive nuclei - Standard for measuring adsorption of material into planar metal surfaces or onto surface of metal nanoparticles |
|
|
Discribe covalent bonding. |
Atoms share their electrons so that each electron shell has inert gas structure
- Very stable interaction that requires large amounts of energy to break - Characteristic geometries |
|
|
Describe ionic bonding. |
Atoms donate/accept electrons from each other in order to acquire full valance shell
Due to electrostatic attraction between two oppositely charged ions
Usually between metal (cation) and non-metal (anion) |
|
|
Describe metallic bonding. |
Share "free" electrons among a lattice of positively charged ions (cations) - Metals generally go into solution as positively charged ions - Metals in general are electron deficient |
|
|
Name the primary and secondary types of bonding. |
Primary: covalent, ionic, metallic
Secondary: hydrogen, Van der Waals, dipole dipole
??? |
|
|
Describe Van der Waals forces. |
Weak bond between molecules due to electric dipoles
?? |
|
|
Describe hydrogen bonds. |
Special dipole-dipole interaction between a (-) charge hydrogen atom and an electronegative atom such as oxygen
???? |
|
|
What situations do you want to use or avoid coating? |
Coating used to create minimally reactive surface by creating an interface that is invisible to system or one that is specifically activated to control cell behaviour at interface
Could decrease adsorption, which could be bad in some cases (alter polarity, charge, etc.) |
|
|
Name and describe a protein resistant surface. |
PolyEthylene Oxide (PEO): highly mobile, hydrophilic polymer that can be grafted onto a surface (or protein) to render resistance to adsorption - Effective way to control complement and coagulation activation |
|
|
Name the three categories of biomolecules, their polymers and constitutive monomers. |
Category > Polymer > Monomer
Carbohydrates > Polysaccharide > Monosaccharides
DNA and RNA > Polynucleic acids > Nucleotides
Proteins > Polypeptides > Amino acids
Proteins: perform specialized functions
Polysaccharides: long carbohydrate molecules
DNA/RNA: storage form of genetic materials |
|
|
Describe the differences between the three molecules of life. |
37, 39, 41-44 |
|
|
What are the categories of amino acids? |
Polar Non-polar Aromatic + charged - charged |
|
|
Properties of nucleic acids and functions. |
? |
|
|
Name three main polysaccharides. |
Cellulose - found in cell walls
Starches - energy storage devices
Chitin - structural carbohydrate found in exoskeletons of insects, spiders, and crustaaceans |
|
|
What reaction forms amino acids into peptides? |
Addition condensation reaction
Carboxy terminus reacts with N terminus to form a covalent bond |
|
|
What are the four protein structures? |
Primary structure - amino acid sequence
Secondary structure - alpha-helix or beta-pleated sheets
Tertiary structure - interaction between non-adjacent amino acid R group
Quaternary structure - interaction between adjacent polypeptides that make up a single protein (in discrete subunits) |
|
|
What happens when the amino acids aggregate more? |
? |
|
|
What reactions tell the protein structures apart? |
? |
|
|
What are nucleic acids? |
Monomers of polynucleic acids that make up DNA and RNA
Consist of three components: sugar, nitrogenous base, and phosphate |
|
|
What is the primary difference between DNA and RNA? |
DNA (deoxyribonucleic acid): encodes genetic instructions used in development and function of all known living organisms with the exception of RNA based viruses - Has a phosphorous backbone
RNA (ribonucleic acid): encodes genetic information
Messenger RNA: carries genetic information that directs synthesis of proteins
Non coding RNAs include transfer RNA and ribosomal RNA
|
|
|
Where is DNA stored? RNA? |
? |
|
|
What is the central dogma of biology? |
SEE PICTURE |
|
|
What are lipids? |
Constitute a broad range of naturally occurring biomolecules that include: fats, waxes, sterols, fat-soluble vitamins (vitamins A D, E, and K), monoglycerides, diglycerides, triglycerides, phospholipids, and others
Lipid monomers are less persistent than others
- Less-readily described using the same polymeric terms - Carbon-rich polymers - Saturated or unsaturated - Make up part of the cell membrane
Main biological functions: - Energy storage - Biomembranes - Cell signalling
|
|
|
What is the difference between a saturated and unsaturated lipid? |
Saturated: no double bonds
Unsaturated: double or triple C=C bonds, causing kinks in chain
Melting point increases with chain length and decreasing double bonds |
|
|
Name the three parts of a phospholipid. |
Phosphate hydrophilic head, glycerol middle, and two hydrophobic fatty acid tails |
|
|
What are some structures that phospholipids can make? |
1. Lipid bilayer 2. Liposome 3. Micelle surrounded by water |
|
|
What functions does the nucleus have and where is it located? |
Controls all activities of the cell: DNA maintenance, RNA transcription
Loc? |
|
|
What functions does the endoplasmic reticulum have and where are they located? |
Translation and folding of new proteins (rough ER), expression of lipids (smooth ER) |
|
|
What functions does the Golgi apparatus have and where is it located? |
Sorting and modification of proteins
loc? |
|
|
What functions do mitochondria have and where are they located? |
Energy production from oxidation of food substances and release of adenosine triphosphate
Chloroplasts play this role in plant cells
loc? |
|
|
What functions does a vacuole have and where are they located? |
Storage of molecules, help maintain homeostasis
loc? |
|
|
What is tissue? What are the four types and where are they located? |
Tissue: formed when cells with the same characteristics or specializations are grouped together
Epithelial - Skin and line hollow organs - Some secretory, some have cilia or protective functions, can form receptors
Connective - Support and reinforce other tissues, in extracellular matrix comprised of proteins (collagen, elastin) - Bones, cartilage, adipose tissue, blood, fibrous connective tissue, loose connective tissue
Muscle - Most abundant tissue type
Nervous - Process info |
|
|
What types of tissue regenerate faster and why? |
High capacity: - epithelial - lymphoid - hematopoietic mesenchymal tissues (fibroblasts, smooth muscle, osteoblasts, chrondrocytes, and endothelial cells)
Low capacity: - nerve - muscle (skeletal and cardiac) - cartilage |
|
|
What are organ systems? Name them. |
Organ system: controlled and regulated body of tissue working together to achieve constancy in the internal environment of the organism.
"Muscular L. CRUISENERD":
Muscular Lymphatic (including immune system) Circulatory Respiratory Urinary Integumentary (like skin) Skeletal Excretory Nervous Endocrine Reproductive Digestive |
|
|
What is wound healing? |
The process of repair
A cascade of events (in a continuum) that involves the interaction of various cellular and molecular components that act in synchrony to effect wound closure by forming new tissue |
|
|
In the wound healing cascade, what is the first to arrive?*** |
Neutrophiles! A granulocyte
- Stick to capillary endothelium, penetrate between endothelial cells and move into surrounding damaged tissue
- Neutrophil emigration beings minutes to hours after insult, may continue as long as 24h
- Activates when engages foreign particle (damage cell, pathogen, damaged matrix, biomaterial) |
|
|
How does wound healing proceed with increasing age? |
It becomes slower, with increased scarring |
|
|
What is the sequence of events following device implantation?*** |
1. Injury - injection, implantation, blood vessel damage 2. Acute inflammation - polymorphonuclear leukocytes 3. Chronic inflammation - monocytes and macrophages 4. Granulation tissue - fibroblasts and new blood capillaries 5. Foreign body reaction - macrophages and FBGCs at the material-tissue interface 6. Fibrous encapsulation - fibrous capsules
IACGFF |
|
|
How long does the sequence of events in wound healing take? |
Acute inflammation - minutes to days
Granulation tissue - occur 3-5 days after implantation
Fibrous encapsulation - occur 4+ weeks after implantation
?????? |
|
|
What happens when a material is not coated? |
? |
|
|
What materials need to be coated? |
? |
|
|
What is hemostasis? |
Process of blood clotting and then subsequent dissolution of clot, following repair of injured tissue
Composed of 4 major events that occur in a set order following loss of vascular integrity |
|
|
What are the four major events that occur to regain hemostasis after loss of vascular integrity? |
1. Vascular constriction - limits the flow of blood to the area of injury
2. Thrombin activate platelets => aggregate at and form loose platelet plug. - Fibrinogen also primarily responsible for stimulation platelet clumping - Platelets clump by binding to collagen that becomes exposed following rupture of endothelial lining of vessels - Platelets release ADP and TXA2, serotonin, phospholipids, lipoproteins, etc. - Additional platelets attract.
3. Fibrin mesh (clot) forms and entraps plug
4. Plasmin causes dissolution of clot to occur for normal blood flow, following tissue repair |
|
|
What are platelets activated by? |
Thrombin - which also activates Protein C |
|
|
What is the primary responsibility of fibrinogen? |
Stimulate platelet clumping
Platelets clump by binding to collagen that becomes exposed following rupture of endothelial lining of vessels |
|
|
What are the three classes of devices? |
Class I: minimal invasiveness, does not contact user internally
Class II: higher degree of invasiveness and risk, but relatively short duration
Class III: considerably more invasive and can pose immense risk to user-implantables |
|
|
What does biocompatable mean? |
Biomaterial, device, or construct can be brought into direction contact with living tissue without: - causing a harmful tissue reaction (pain, swelling or necrosis) that could compromise function - causing a systemic toxic reaction - having tumorigenic potential
i.e. The ability of a material to perform with an appropriate host response in a specific application |
|
|
What are some application examples of metals? |
Cobalt-chronium alloys: heart valves, dental prostheses, orthopedic fixation plates
Gold and platinum: dental fillings
Stainless steel: dental prosthesis, orthopedic fixation plates |
|
|
What is a disadvantages of metal implants? |
Can cause stress shielding - implant will take the load, decreasing the stress on the actual joint, which can lead to lower bone density
The healthy bone tissue will remodel in response to the loads it is placed under - Can cause complications at tissue/implant interface |
|
|
What if face centered cubic structure? |
- Atoms are located at corners of unit cell and in centers of each face
- Equation relating radius to edge length is:
a=2r*sqrt(2) |
|
|
What is body centered cubic structure? |
- Corner atoms are shared by 8 unit cells and there is one atom in the middle
a = 4r/sqrt(3) |
|
|
Considering ceramic stability, what is coordination number? |
Coordination number: number of central atom's nearest neighbours
Need a minimum radius ratio Rcation/Ranion
SEE P. ? IN LECTURE 2 FOR IMAGE (TABLE 2.4) |
|
|
What are some classifications of polymers? |
Hydrophilic, hydrophobic, biostable, biodegradable, natural, synthetic, highly processable
Can belong to more than one category |
|
|
What is a main difference between HDPE and LDPE? |
Low density polyethylene has more branches, which increases the volume and thus reduces the density of the polymer |
|
|
What are the different types of polymers and how are they classified? |
Thermosets - processing Thermoplastics - processing Elastomers - mechanical properties Hydrogels - chemical properties Polyelectrolytes - chemical properties Natural - based on origin Biodegradable - based on biostability |
|
|
What makes polymers different from each other? |
Strength of intermolecular forces and sum over long polymer chains
Molecular weight and entanglement, which slow down motion - increases viscosity
Crystallinity (strongly impeded by change entanglement)
Cross-linking |
|
|
What do hydrogen bonds occur between? |
Between hydrogen and F, O, or N (highly electronegative partners) |
|
|
What is the difference between adsorption and absorption? |
Absorption: penetration of molecules into a substance
Adsorption: adhesion of molecules to the surface of a solid substance - Important for biomaterials |
|
|
What governs protein adsorption to surfaces? |
Surface hydrophobicity
Surface charge |
|
|
What are bulk materials described by? |
Chemical/molecular composition
Atomic/molecule structure (crystallinity, etc.)
Mechanics (elasticity, etc.)
Shape |
|
|
Surfaces of materials have which unique descriptive properties? |
Excess surface free energy
Atomic/molecular composition
Chemical composition (reactivity)
Topography (vs. shape)
Surface characterization provides surface specific information about these properties |
|
|
Where does blood coagulate more? |
Blood coagulates more rapidly on negatively charged glass than on hydrophobicaly modified glass or polymers |
|
|
Name an anticoagulant. |
Heparin (negatively charged) |
|
|
*** GET ANSWERS TO EXAMPLES IN LECT 4 |
------- |
|
|
What factor is surface activated? |
Hageman Factor (Factor XII) - adsorption |
|
|
What are carbohydrate molecules joined together by? |
Glycosidic bonds |
|
|
What are sugars held together by? |
Covalent bonds |
|
|
How many amino acids are there? |
20 |
|
|
What determines an organisms phenotype? |
Proteins |
|
|
How are polypeptides and proteins different? |
Protein refers to the biologically active form of a polypeptide |
|
|
What are amino acid made of? |
Amino terminus, radical group, carboxy terminus
NH2 - RCH - COH=O
|
|
|
How do amino acids form peptides? |
Condensation reaction.
Carboxy terminus reacts with N terminus to form a covalent bond, releasing water |
|
|
What makes the peptide bond stable and rigid? |
Resonance of the double bond
Thus, peptide backbone is quite rigid |
|
|
What is a nucleoside? |
Only has the sugar and nitrogen base, as opposed to a nucleotide which also has a phosphate |
|
|
What are pyrimidines and purines? |
Pyrimidines: heterocyclic aromatic organic compounds similar to benzene, containing two nitrogen atoms at position 1 and 3 of 6-member ring - Thymine, Cytosine, and Uracil
Purines: heterocyclic aromatic organic compound, consisting of a pyrimidine ting fused to an imidazole ring - "shorter name, longer molecule" - Adenine and Guanine |
|
|
What is DNA's structure? |
A, C, G, T attached to a sugar-phosphate backbone
Double-helix
Through hydrogen bonding: A=T G=C |
|
|
What is RNA's structure? |
U replaces T
Important when translating DNA to RNA as DNA serves as temple for RNA
mRNA is single-stranded |
|
|
What is transfer RNA? |
Adaptor molecule composed of RNA (73-93 nucleotides)
Bridges genetic code (ATGC) in mRNA with 10-letter amino acids in protein
Set of 3 nucleotides called codon refers to specific amino acid to be added |
|
|
How does tRNA work during protein synthesis? |
tRNA bound to amino acid
Combines to matching mRNA on ribosome, connecting previous amino acids to the new one forming growing peptide chain |
|
|
What are the other non coding RNAs? |
Ribosomal RNA - component of ribosome
MircoRNA (miRNA) - short, found in eukaryotic cells - Regulates gene expression - Not well understood |
|
|
What is the difference between fats and oil? |
Fats: - Fully saturated - Solid at room temp - > high-temp stability
Oils: - Unsaturated - Liquid at room temp - >low-temp fluidity |
|
|
What is cholesterol? |
A membrane temperature fluidity buffer |
|
|
Describe normal tissue. |
Multi-cellular 3D structures Extracellular matrix Multi-functional Takes cues from environment Interface with surroundings |
|
|
Name the potential results of tissue injury. |
- Necrosis - death by extrinsic means
- Apoptosis - death by suicide
- Atrophy - decrease in cell size/function
- Hypertrophy - increase in cell size
- Hyperplasia - increase in cell numbers
- Metaplasia - change in cell type
- Change in phenotype - change in type and/or amount of protein characteristic of particular cell type |
|
|
What is necrosis? |
Cell death by extrinsic means e.g. infection, toxin, trauma that result in unregulated digestion of cell components |
|
|
What is apoptosis? |
Programmed cell death via suicide - Occurs in a series of steps |
|
|
What is atrophy? |
Decrease in cell size and/or function
Causes include mutations, poor nourishment, poor circulation, loss of hormonal support, loss of nerve supply to target organ, excessive amount of apoptosis of cells, and disuse/lack of exercise |
|
|
What is hypertrophy? |
Increase in cell size (not number) |
|
|
What is hyperplasia? |
Increase in cell numbers (not size) |
|
|
What is mataplasia? |
Reversible replacement of one differentiated cell type with another mature differentiated cell type |
|
|
What is acute inflammation associated with? |
Polymorphonuclear leukocytes |
|
|
What is chronic inflammation associated with? |
Monocytes and macrophages |
|
|
What is granulation tissue associated with? |
Fibroblasts (which produce ECM and differentiate into myofibroblasts) and new capillaries
Granulation tissue: ECM, fibroblasts, and new blood vessels |
|
|
What is the foreign body reaction associated with? |
Macrophages and FBGCs at the material-tissue interface |
|
|
What is fibrosis associated with? |
Fibrous capsule |
|
|
Briefly describe hemostasis: vasoconstriction and plug formation. |
1. Exposed collagen binds and activates platelets 2. Release of platelet factors 3. Attracts more platelets 4. Aggregate into platelet plug |
|
|
What initiates the wound healing process? |
Platelets bind to matrix and aggregate to form temporary plug - Secretion of small soluble molecules from cytoplasmic granules called growth factors and cytokines - Platelet derived growth factor (PDGF), fibronectin, von Willebrand Factor, Transforming Growth Factor-beta (TGF-b) |
|
|
What is another word for fibrin clot formation? |
Thrombogenesis |
|
|
What are the two principle pathways of thrombogenesis? |
Intrinsic pathway: clot in response to abnormal vessel wall superficial injury in adsense of tissue injury
Extrinsic pathway: clot formation in response to tissue injury, actual breakage of blood vessels
Both pathways converge on the same end product - fibrinogen fibrin.
Both also involve numerous proteolytic enzymes called clotting factors |
|
|
What are Zymogens? |
Enzyme precursors that are not active.
Become a [##]a when active. |
|
|
What is the difference between the intrinsic and extrinsic pathway? |
Intrinsic is slower - few seconds to minutes to produce Factor X
Extrinsic reacts almost instantaneously but produces less - Augments intrinsic pathway by slowing blood flow |
|
|
What can activate the complement system? What is the complement system? |
Blood-materials interactions that lead to adsorption
Complement system is a complex cascade involving ~30 glycoproteins present in serum and cell surface receptors
Part of the innate immune system
Activation of inflammation and immune related function |
|
|
What are the types of cytokines and growth factors? |
Autocrine - affect function of the cell that releases it
Paracrine - affect function of adjacent or nearby cells of same or different phenotype |
|
|
Compare granulocytes, leukocytes, and macrophages. |
Granulocyte: any blood cell containing specific granules (neutrophils, eosinophils, basophils)
Leukocyte: colourless blood cell capable of ameboid movement (lymphocytes, monocytes, granulocytes)
Macrophage: large phagocytic mononuclear cell |
|
|
What is von Willenbrand Factor? |
A protein (large adhesive glycoprotein) produced by endothelial cells
Stabilizes Factor VII and essential for platelet adhesion |
|
|
What is heparin? |
Polysaccharide that enhances activity of antithrombin III - a protein inhibitor enzyme of thrombin
However, not effective against Fibrin-bound thrombin - role is thus to limit thrombin activity away from site of clotting |
|
|
What is Protein C? |
Activated by thrombin
Serine protease - initial step in dismantling blood clotting cascade
Targets are non0enzymatic cofactors - Factor V and VIII
-> growth of new clot material is slowed and eventually stopped
Genetic defects result in thrombosis - severe, recessive form that results in neonatal death |
|
|
What do you call growth of new blood vessels? |
Angiogenesis - Induced by low O2 levels, low pH, elevation of cytokines |
|
|
How does FBR depend on geometry and form of the implant? |
FBR = foreign body reaction cells (giants)
Flat and smooth surface - 1-2 macrophages cells thick
Relatively rough surface - multiple layers of FBGC and macrophages
Rough surface - macrophages and FBGC with varying degrees of granulation tissue |
|
|
What are the possible outcomes for the implant? |
Resorption: implant site eventually resolves to a collapsed scar or may completely disappear (bone)
Integration: very limited occurrence; approximates normal host tissue without intervening capsule
Encapsulation: most usual response |
|
|
What part of the immune system involves inflammation? |
Innate immune system |
|
|
What happens in inflammation and what are some symptoms? |
1. increased vascular permeability 2. infiltration of blood plasma proteins and leukocytes 3. opsonization and phagocytosis of foreign material
Swelling, edema, heat, pain |
|
|
What are the differences between the innate and adaptive immune system? |
Innate: non-specific, no memory, first line of defense against foreign pathogens - DOES NOT CHANGE OVER COURSE OF INDIVIDUALS LIFETIME
Adaptive: specific, involves highly specialized systemic cells and processes that prevent pathogenic growth |
|
|
What are the two immune systems? |
Innate and adaptive |
|
|
How does adaptive immune system recognize foreign material? |
Antibodies (aka immunoglobulins)
IgG is most common |
|
|
What is part of the innate immune system? |
Complement and leukocytes |
|
|
What is opsonin? |
Any molecule that targets an antigen for an immune response |
|
|
What is the complement system? |
"Complements" the ability of antibodies and phagocytic cells to clear pathogens from an organism |
|
|
What are the three complement pathways? |
Alternative: binding of complement proteins to microbial cell surface - Components are abundant in serum
Classical: binding of complement proteins to antibody (C1 activation) - Most of pathway feeds into alternative pathway
Both cause formation of C3 convertase, which cleave C3 and continue down pathway
Lectin: like classical pathways but with opsonin, mannose-binding lectin (proteins that bind sugars) and ficolins
All cause C3a inflammation, opsonization and phagocytosis, C5a inflammation, and lysis of microbe |
|
|
What are the general features of the complement system? |
Amplification, solid-state, soluble signals, multiple inhibitors |
|
|
What would lack of complement cause? Overactive complement? |
Lack: recurring bacterial infection
Overactive: worse fate; Alzheimer's disease, etc. |
|
|
What are the three major roles of complement? |
1. Opsonize particles for phagocytosis
2. Elicit inflammatory reaction by acting on leukocytes, mast cells, endothelium
3. Complement-mediated cytolysis |
|
|
What are the primary effectors of the innate immune system? |
Monocytes (macrophages) |
|
|
What are some important contributing factors to issues with biomaterials devices? |
Plasma protein adsorption Complement response Fibrinogen Mast cells/histamine particulate size and concentration TNF-alpha Motion |
|
|
What are some infectious agents? |
Bacteria Viruses Fungi Animal parasites |
|
|
What is an infection acquired in a hospital called? |
Nosocomial caused primarily from bacteria during device insertion or implantation procedure |
|
|
What is cell culture? And why do we need it? |
Complex process by which cells are grown under controlled conditions
Investigate normal physiology or biochemistry of cells, test effect of various chemical compounds or drugs on specific cell types, synthesize valuable biological agent form large scale cell cultures, use various cell types to engineer artifical tissues |
|
|
What is the significance of HeLa? |
Cell line derived from cerival cancer in Henrietta Lacks
First "immortal" human cell line - constantly expresses telomerase |
|
|
What is the difference between primary and immortal cell lines? |
Primary: often contain a variety of cell types; only passage a certain number of times before die
Immortalized: possess ability to proliferate indefinitely either through deliberate modification or random mutation |
|
|
What are some components of cell culture media? |
Amino acids, salts, glucose, vitamins, phenol red for pH indicator, antibiotics, growth factors
SERUM |
|
|
What is serum? |
Clear yellowish fluid obtained upon separating whole blood into solid and liquid components after allowed to clot |
|
|
What are two different cell cultures? |
Adherent: must attach to surface to survive/proliferate
Non-adherent: can be grown in suspension culture |
|
|
What is passing? |
Technique that enables an individual to keep cells alive and growing under cultured conditions for extended periods of time - Must dissociate cells from plate using enzymes such as tryspin or collagenase for adherent cells - Must dilute cells in fresh media and replate |

